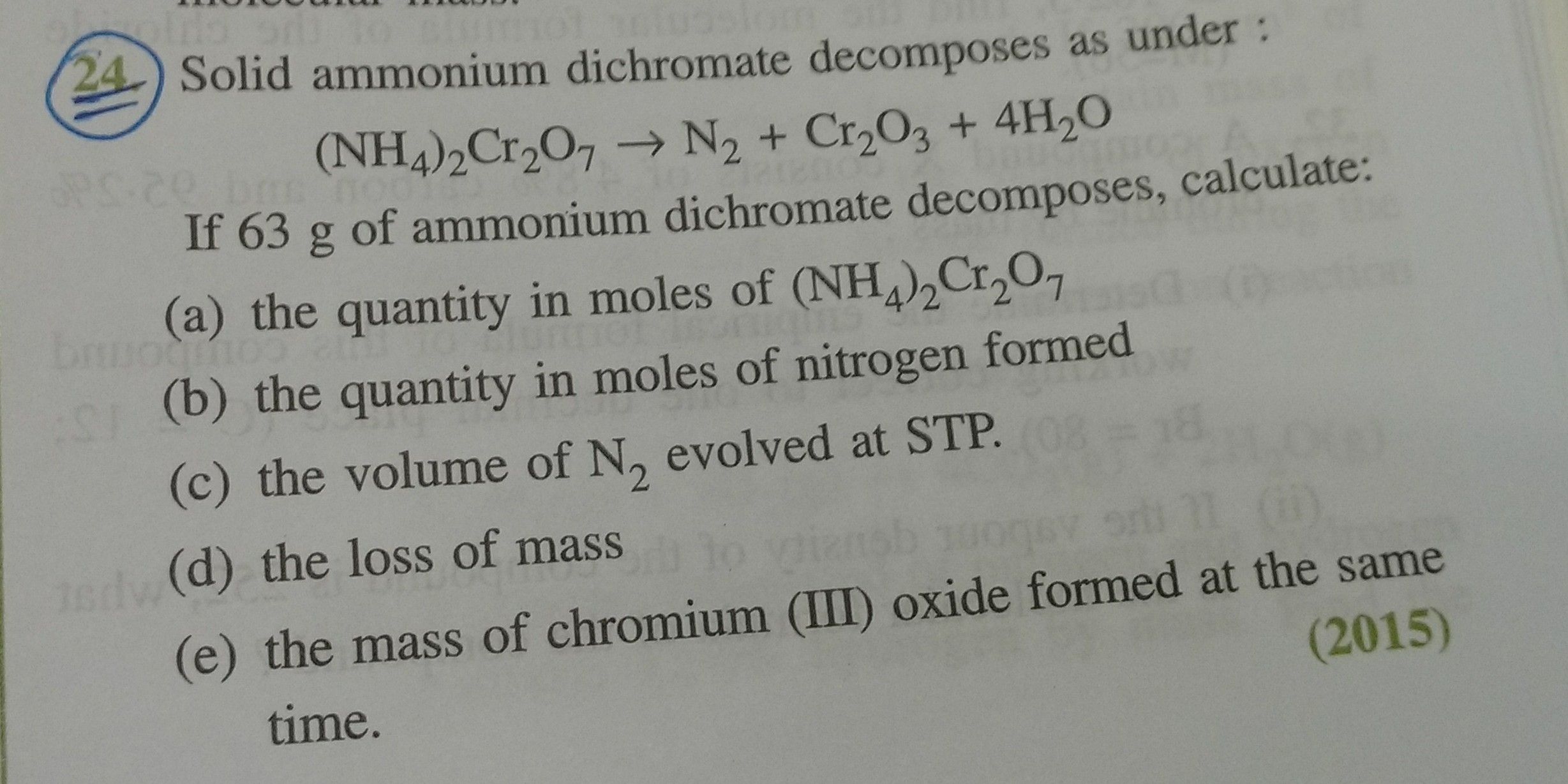ICSE Class 10 Answered
State why the atomic weight of an element is also termed as relative atomic mass
Asked by mkp400 | 03 Jan, 2018, 07:58: PM
Atoms being extremely small, cannot be seen or weighted. But by using indirect methods of physics we can calculate absolute mass of all kinds of atoms.
As these masses are too small, it is not convenient touse kilograms or grams as a unit.
Therefore, it is more appropriate to use mass of some standard atom as a unit and then relate the masses of other atoms to it.
Thus resulting atomic masses are called as 'relative atomic masses.'
Answered by Varsha | 04 Jan, 2018, 11:17: AM
Application Videos
Concept Videos
ICSE 10 - Chemistry
Asked by maybe.kushagra | 25 Jan, 2024, 03:12: AM
ICSE 10 - Chemistry
Asked by srinu2020.ravipati | 16 Sep, 2020, 03:33: PM
ICSE 10 - Chemistry
Asked by Gurdev71 | 24 Jun, 2020, 12:41: PM
ICSE 10 - Chemistry
Asked by Kanwaranita10 | 16 Feb, 2020, 11:22: AM
ICSE 10 - Chemistry
Asked by aashimegh | 17 Aug, 2019, 02:24: PM
ICSE 10 - Chemistry
Asked by aashimegh | 03 Aug, 2019, 11:50: AM
ICSE 10 - Chemistry
Asked by Shrinivasdangi07 | 21 Mar, 2019, 10:34: PM
ICSE 10 - Chemistry
Asked by johncena9384 | 26 Oct, 2018, 04:08: PM
ICSE 10 - Chemistry
Asked by yajay0441 | 27 Aug, 2018, 03:15: PM