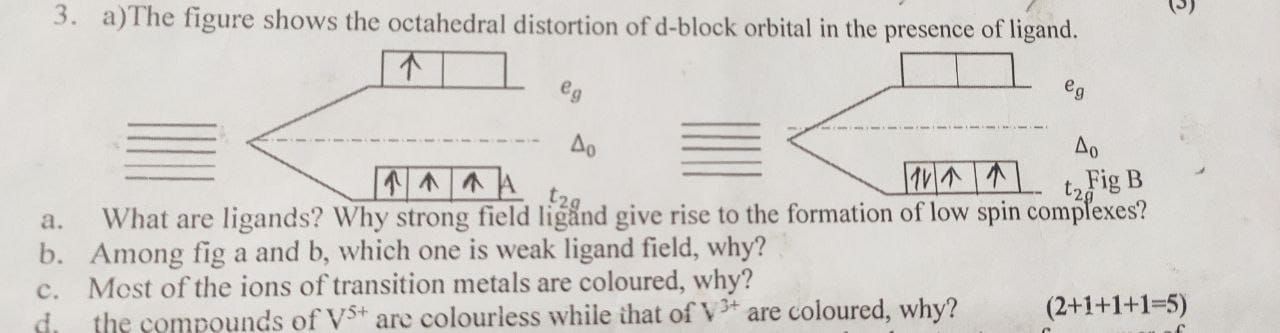
CBSE Class 12-science Answered
There are several factors that affect stability of complex ion. One of them is formation of chelate rings. If L is unidentate ligand and L-L, a bidentate ligand and if the donor atoms of L and L-L are same element then compound formed with L-L ligand is more stable than formed with L ligand. In general ring provides stability to complex. The chelate effect describes the enhanced affinity of chelating ligands for a metal ion compared to the affinity of a collection of similar nonchelating (monodentate) ligands for the same metal. Chelated complex are more stable than similar complexes with unidentate ligand as dissociation of complex involves breaking two bonds rather than one.