CBSE Class 12-science Answered
Solve it.
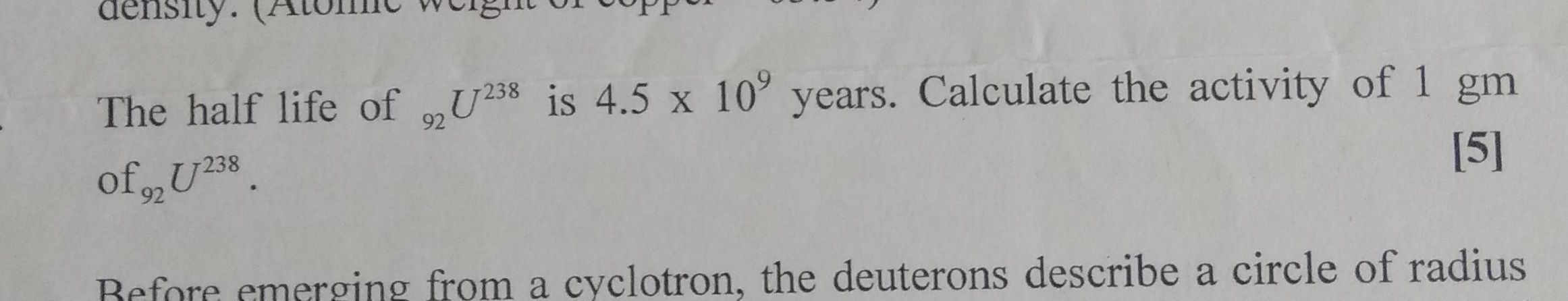
Asked by sahupramod650 | 19 Nov, 2019, 10:36: AM
Relation involving half life T1/2 and decay constant λ is given by, λ =0.693 / T1/2 .............(1)
hence decay constant λ for 238U92 = 0.693 /( 4.5 × 109 × 365 × 24 × 3600 ) = 4.883 × 10-18 s-1
activity , dN/dt = λN ...............(2)
where N is number of atoms in 1 gm Uranium, N = 6.02 × 1023 / 238 = 2.529 × 1021
activity = 4.883 × 10-18 × 2.529 × 1021 = 12.35 × 103 Bq = 12350 Bq
( 1 Bq = i disintegration per sec )
activity in Curie = 12350/(3.7 × 1010 ) = 3.34 × 10-7 Ci = 0.334 μCi
Answered by Thiyagarajan K | 19 Nov, 2019, 12:24: PM
Concept Videos
CBSE 12-science - Physics
Asked by artabandhusahu85 | 24 Apr, 2024, 12:07: PM
CBSE 12-science - Physics
Asked by niharvijayvargiya5 | 23 Apr, 2024, 06:40: PM
CBSE 12-science - Physics
Asked by kulhariabhijeet | 21 Apr, 2024, 02:39: PM
CBSE 12-science - Physics
Asked by mohapatraswetalina88 | 21 Apr, 2024, 12:18: PM
CBSE 12-science - Physics
Asked by aishaisha091098 | 19 Apr, 2024, 04:54: PM
CBSE 12-science - Physics
Asked by dasrituparna1999 | 13 Apr, 2024, 06:56: AM
CBSE 12-science - Physics
Asked by dasrituparna1999 | 12 Apr, 2024, 09:26: PM
CBSE 12-science - Physics
Asked by mishrigupta19319 | 08 Apr, 2024, 06:28: PM
CBSE 12-science - Physics
Asked by madhav9119887644 | 07 Apr, 2024, 08:10: PM