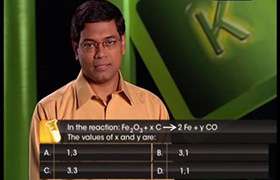CBSE Class 11-science Answered
Solve it

Asked by g_archanasharma | 22 Mar, 2018, 10:11: PM
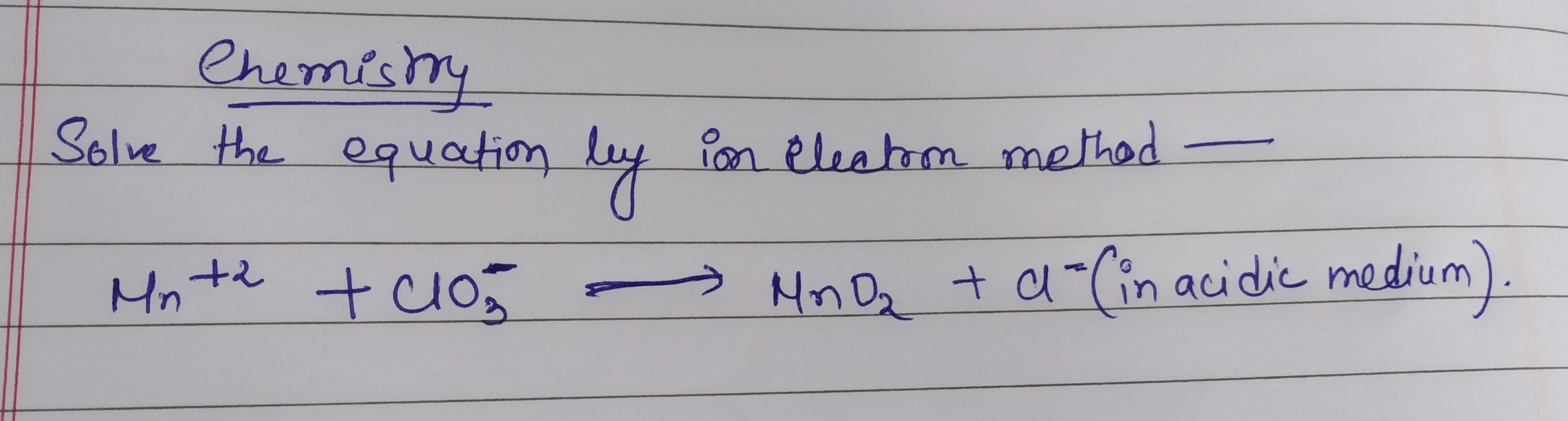
For balancing a redox reaction, you have to balance the no. of atoms on reactant and product side i.e, no. of atoms on both sides should be equal.

no.of Hydrogen atoms are not equal on both sides.

In CuO the oxidation no. of Cu is +2 while in Cu its 0 that means Cu gets reduced,
and in NH3 Oxidation no of N is -3 and in the product, it produced N2 with 0 oxidation no. hence it gets oxidised
There is no change in the O.S. of oxygen. lets balanced it by multiplying CuO by 3 we get,


Answered by Ramandeep | 23 Mar, 2018, 09:26: AM
Concept Videos
CBSE 11-science - Chemistry
Asked by indranibaudya | 03 Oct, 2020, 08:12: PM
CBSE 11-science - Chemistry
Asked by Punshibakhuraijam2015 | 22 Sep, 2019, 08:40: PM
CBSE 11-science - Chemistry
Asked by kkdmmdsd | 09 Jun, 2019, 04:27: PM
CBSE 11-science - Chemistry
Asked by dheerajmathpal374 | 15 Sep, 2018, 10:43: AM
CBSE 11-science - Chemistry
Asked by govtsecschoolnayaganv051 | 19 Aug, 2018, 06:29: PM
CBSE 11-science - Chemistry
Asked by govtsecschoolnayaganv051 | 18 Aug, 2018, 05:08: PM
CBSE 11-science - Chemistry
Asked by govtsecschoolnayaganv051 | 14 Aug, 2018, 06:06: PM
CBSE 11-science - Chemistry
Asked by vaagai2353 | 29 Jun, 2018, 10:29: PM
CBSE 11-science - Chemistry
Asked by g_archanasharma | 22 Mar, 2018, 10:11: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM