CBSE Class 11-science Answered
sir,
why orthorombic solid have 3 non primitive structure whereas monoclonic do not ,even both have all unside unequal? explain with diagram.
Asked by lucky170313 | 20 Mar, 2015, 07:08: AM
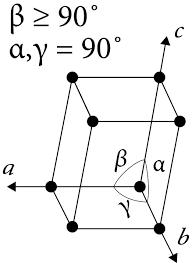
There is one major difference between orthorhombic and monoclinic structure.
In orthorhombic structure, all the angles are orthogonal that means α=β=γ=90 °C.
While in case of monoclinic structure, α=γ=90 °C but β≠90 °C.
The diagram for the monoclinic lattice is as follows:
Answered by Prachi Sawant | 21 Mar, 2015, 07:51: PM
Concept Videos
CBSE 11-science - Chemistry
Asked by kamalpavenkp123 | 11 Mar, 2024, 02:49: PM
CBSE 11-science - Chemistry
Asked by akankhyapradhan123 | 16 Jan, 2022, 07:46: AM
CBSE 11-science - Chemistry
Asked by Amit176039 | 03 Oct, 2020, 03:21: PM
CBSE 11-science - Chemistry
Asked by shubhamanand1369 | 26 May, 2020, 11:38: AM
CBSE 11-science - Chemistry
Asked by amangeneralstore27 | 21 Dec, 2019, 11:25: AM
CBSE 11-science - Chemistry
Asked by atulpd | 29 Mar, 2018, 10:42: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 08 Oct, 2014, 03:00: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 08 Oct, 2014, 03:01: PM