CBSE Class 10 Answered
sir
Samples of four metals A, B, C and D were taken and added to the following solution one by one. The results obtained have been tabulated as follows.
Use the table above to answer the following questions about metals A, B, C and D.
(i) Which is the most reactive metal?
(ii) What would you observe when metal B is added to a solution of copper (II) sulphate?
(iii) Arrange the metals A, B, C and D in the order of decreasing reactivity.
| Metal | Iron (II) sulpate | Copper (II) sulpate | Zinc sulphate | Silver nitrate |
| A | No reaction | Displacement | ||
| B | Displacment | No reaction | ||
| C | No reaction | No reaction | No reaction | Displacemet |
| D | No reaction | No reaction | No reaction | No reaction |
(i) Which is the most reactive metal?
(ii) What would you observe when metal B is added to a solution of copper (II) sulphate?
(iii) Arrange the metals A, B, C and D in the order of decreasing reactivity.
Asked by seeni2005 | 24 Jul, 2016, 03:50: PM
From the given table we can write following observations:
1) A is less reactive than Fe but more reactive than Cu.
2) B is more reactive than Fe but less reactive than Zn.
3) C is more reactive than Ag but less reactive than Fe, Cu and Zn.
4) D is less reactive than Fe, Cu, Zn, Ag.
Hence we can conclude that,
(i) B is the most reactive metal.
(ii) B can displace Cu as B is more reactive than Cu.
(iii) The reactivity of these metals in the decreasing order is B > A > C > D.
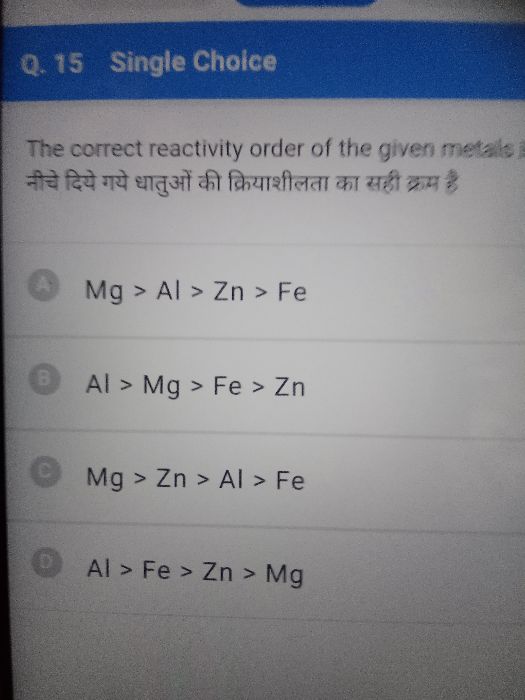
Reactivity series,
Zn > B > Fe > A > Cu > C > Ag > D
Answered by Prachi Sawant | 24 Jul, 2016, 10:58: PM
Application Videos
Concept Videos
CBSE 10 - Chemistry
Asked by ritik9897022 | 05 Feb, 2024, 09:42: PM
CBSE 10 - Chemistry
Asked by shubham.sharma80634 | 05 Oct, 2021, 09:18: AM
CBSE 10 - Chemistry
Asked by virkarman36 | 08 Aug, 2021, 09:24: AM
CBSE 10 - Chemistry
Asked by dnupadhyay214 | 13 Mar, 2021, 12:01: PM
CBSE 10 - Chemistry
Asked by Vishavjet567 | 31 Oct, 2020, 10:52: AM
CBSE 10 - Chemistry
Asked by aryanluniwal1516 | 12 Sep, 2020, 11:43: AM
CBSE 10 - Chemistry
Asked by broprint18 | 07 Jun, 2020, 04:16: PM
CBSE 10 - Chemistry
Asked by prakharsingh167 | 25 May, 2020, 10:20: PM
CBSE 10 - Chemistry
Asked by sonaliagarwal172 | 16 May, 2020, 10:16: AM