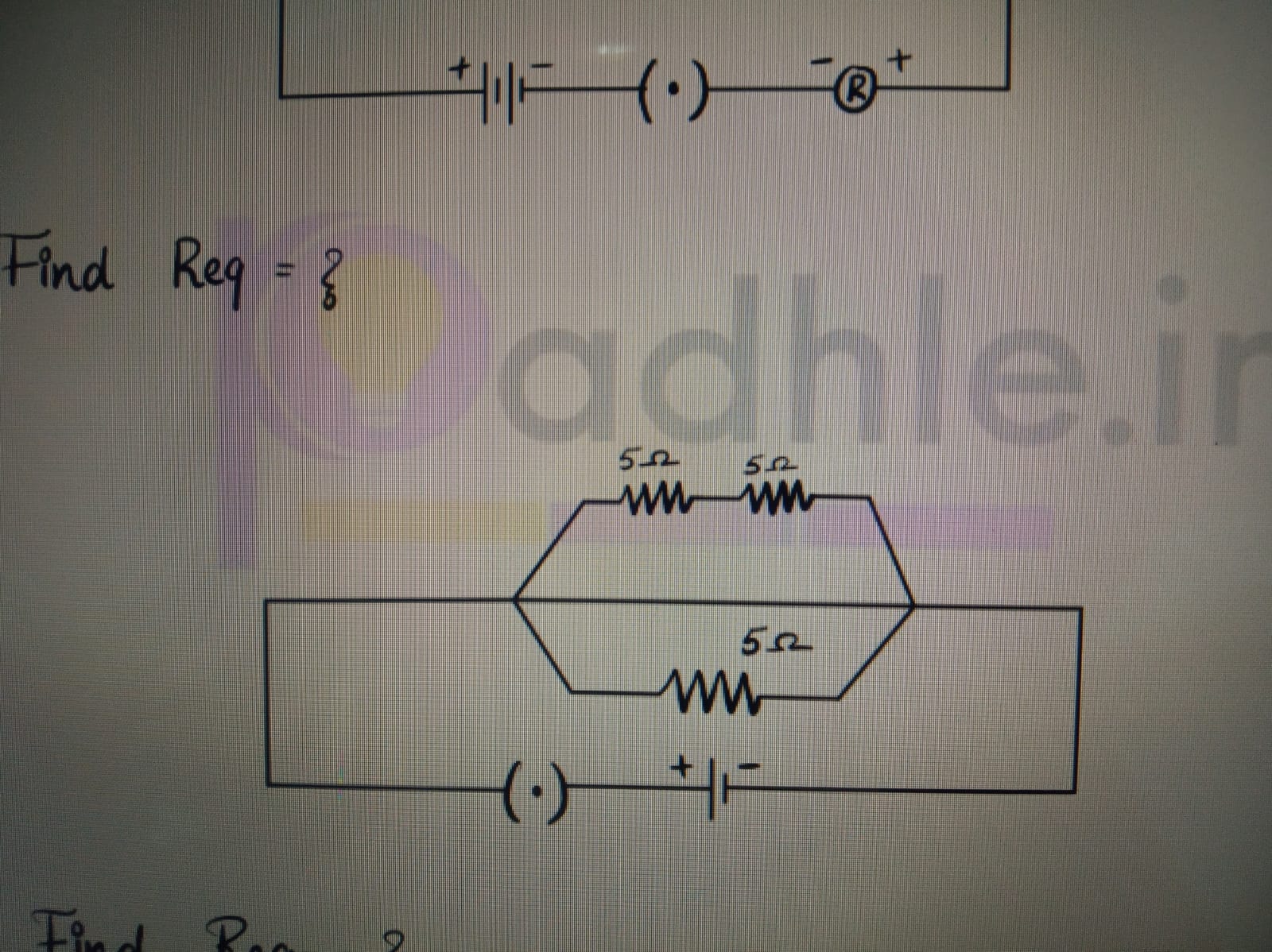
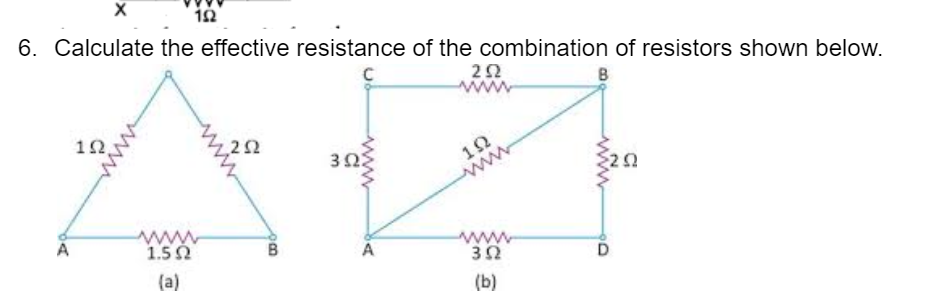
CBSE Class 10 Answered
Sir,regarding Joules' law of heating somewhere in the book, it is written H=I^2 Rt and somewhere it is written H=VIt/4.2 cal. It is right that IR is substituted for V.
But, Sir please note that I have a calorimeter(Joules apparatus)with a thermometer.
But the thermometer will give reading in celsius. So please tell me how to change celsius in calorie or joule. It is also written on the calorimeter:1 volt,6 amp. What does this mean, I do not understand. Also please tell me why in the formula, it is written 4.2 cal. Please give full explanation about how to carry out this experiment.
Asked by Manoj | 19 Jun, 2013, 07:42: PM
Pls post one query at a time.
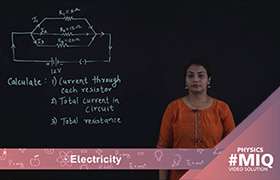
Amount of heat, Q = Mass x specific heat x change in temperature
H = I2Rt Joule
1 cal = 4.2 J
So, H = (I2Rt)/4.2 cal
= (VIt)/4.2 cal
Answered by | 19 Jun, 2013, 11:04: PM
Application Videos
Concept Videos
CBSE 10 - Physics
Asked by khajannirwan | 27 Feb, 2024, 10:20: PM
CBSE 10 - Physics
Asked by saanviyadla | 24 Jan, 2024, 07:06: PM
CBSE 10 - Physics
Asked by kamalaranjanmohantymohanty5 | 06 Jan, 2024, 10:05: AM
CBSE 10 - Physics
Asked by nandhikasugumar | 05 Oct, 2023, 04:01: PM
CBSE 10 - Physics
Asked by daniya062008 | 02 Oct, 2023, 08:25: PM
CBSE 10 - Physics
Asked by prassanna.j | 03 Sep, 2023, 12:28: PM
CBSE 10 - Physics
Asked by prassanna.j | 03 Sep, 2023, 12:21: PM
CBSE 10 - Physics
Asked by prassanna.j | 03 Sep, 2023, 12:13: PM
CBSE 10 - Physics
Asked by prassanna.j | 03 Sep, 2023, 12:11: PM