CBSE Class 11-science Answered
Sir/madam
I have a question. How can we say that water spreads on glass easily inspite of its low surface tension. Also how can we say that the interface of mercury-glass does not spread on glass easily inspite of its high surface tension?????plz reply quickly!!!
Asked by shravan s | 02 Dec, 2013, 11:48: AM
How easily a liquid drop spreads on a solid surface depends on contact angle.
A contact angle less than 90° (low contact angle) usually indicates that wetting of the surface is very favorable, and the fluid will spread over a large area of the surface. Contact angles greater than 90° (high contact angle) generally means that wetting of the surface is unfavorable so the fluid will minimize contact with the surface and form a compact liquid droplet.
The angle of contact between water and glass typically lies in the range 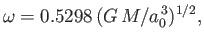 to
to 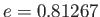 , whereas that between mercury and glass is about
, whereas that between mercury and glass is about 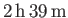
Therefore, mercury does not spread easily on glass surface while water spreads easily on it.
|
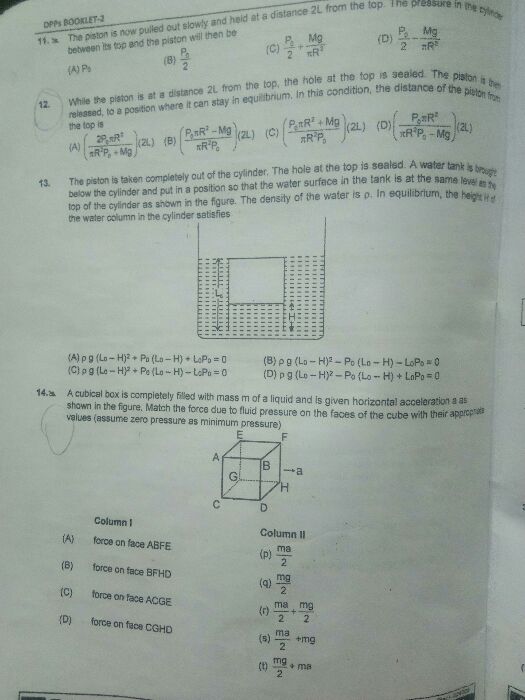
Contact angle |
Degree of wetting |
|
θ = 0 |
Perfect wetting |
|
0 < θ < 90° |
high wettability |
|
90° ≤ θ < 180° |
low wettability |
|
θ = 180° |
perfectly |
Answered by Komal Parmar | 02 Dec, 2013, 03:02: PM
Concept Videos
CBSE 11-science - Physics
Asked by rajuinwati12 | 04 Mar, 2024, 09:22: AM
CBSE 11-science - Physics
Asked by joelisaacsam | 09 Jan, 2024, 06:31: AM
CBSE 11-science - Physics
Asked by rupimadhes | 21 Nov, 2023, 05:31: AM
CBSE 11-science - Physics
Asked by arjunsah797 | 12 Mar, 2022, 11:10: AM
CBSE 11-science - Physics
Asked by suragimathpraveen2001 | 10 Jan, 2022, 08:06: PM
CBSE 11-science - Physics
Asked by raghuvanshisaumya461 | 03 Dec, 2021, 08:05: AM
CBSE 11-science - Physics
Asked by abhinavakrishna1009 | 31 Mar, 2021, 11:15: AM
CBSE 11-science - Physics
Asked by vanshraaj.ind | 13 Dec, 2020, 06:06: PM
CBSE 11-science - Physics
Asked by ashanihalchandani | 26 May, 2020, 09:11: AM
CBSE 11-science - Physics
Asked by mahimanaik290 | 10 May, 2020, 12:45: AM