CBSE Class 10 Answered
sir could you please explain me briefly about the hydronium ions
Asked by Megha | 19 Jun, 2014, 03:37: PM
Hydronium ion (H3O+) is a water molecule having an extra hydrogen ion attached to it. The reaction is as follows:
( H2O + H+→ H3O+).
It is used to determine the acidity of a chemical compound. When a compound is dissolved in water solution, the more the hydronium ion is produced, the higher the acidity is.
For example: HCl(aq)+ H2O(l) 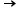 Cl-+ H3O+ . Thus, the Hydrogen ion is transferred to water molecule.
Cl-+ H3O+ . Thus, the Hydrogen ion is transferred to water molecule.
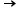 Cl-+ H3O+ . Thus, the Hydrogen ion is transferred to water molecule.
Cl-+ H3O+ . Thus, the Hydrogen ion is transferred to water molecule.
Answered by Prachi Sawant | 20 Jun, 2014, 09:23: AM
Application Videos
Concept Videos
CBSE 10 - Chemistry
Asked by amanazeez6 | 14 Dec, 2023, 01:10: PM
CBSE 10 - Chemistry
Asked by ssyedbasha49 | 16 Jan, 2023, 02:17: PM
CBSE 10 - Chemistry
Asked by murugan3 | 25 Dec, 2022, 06:12: AM
CBSE 10 - Chemistry
Asked by Trisha Gupta | 23 Nov, 2021, 02:40: AM
CBSE 10 - Chemistry
Asked by oyaulakh | 15 Sep, 2021, 06:55: AM
CBSE 10 - Chemistry
Asked by bhavikabhatia1125 | 03 Jul, 2021, 12:16: PM
CBSE 10 - Chemistry
Asked by bhavikabhatia1125 | 02 Jul, 2021, 10:14: PM
CBSE 10 - Chemistry
Asked by vpaishwarya061 | 14 Jun, 2021, 10:34: PM
CBSE 10 - Chemistry
Asked by nityashreebs | 18 Mar, 2021, 07:36: PM
CBSE 10 - Chemistry
Asked by Lanzmik | 04 Nov, 2020, 07:54: AM





