CBSE Class 11-science Answered
Show that the average kinetic energy of a gas molecule is directly proportional to the temprature of the gas. hence give the kinetic interpretation of temprature.
Asked by ashwinikumar59 | 16 Feb, 2011, 07:19: AM
Dear student
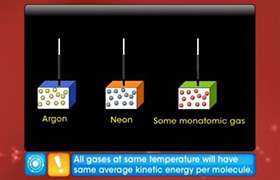
This is the relation between average kinetic energy per molecule of gas and temperature and according to this relation average kinetic energy of a molecule of an ideal gas is proportional to its absolute temperature. This is called kinetic interpretation of temperature.
Hope this helps.
Regards
Team
Topperlearning
Answered by | 16 Feb, 2011, 09:47: AM
Concept Videos
CBSE 11-science - Physics
Asked by ifrayaseen31 | 28 Oct, 2023, 09:26: AM
CBSE 11-science - Physics
Asked by pratikshyadashrkl | 01 May, 2020, 10:24: AM
CBSE 11-science - Physics
Asked by karanchandra34 | 29 Jan, 2019, 11:10: PM
CBSE 11-science - Physics
Asked by Madhurimaurya609 | 11 Jul, 2018, 08:14: PM
CBSE 11-science - Physics
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 11-science - Physics
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 11-science - Physics
Asked by Topperlearning User | 11 May, 2015, 01:59: PM
CBSE 11-science - Physics
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 11-science - Physics
Asked by Topperlearning User | 11 May, 2015, 02:13: PM
CBSE 11-science - Physics
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM