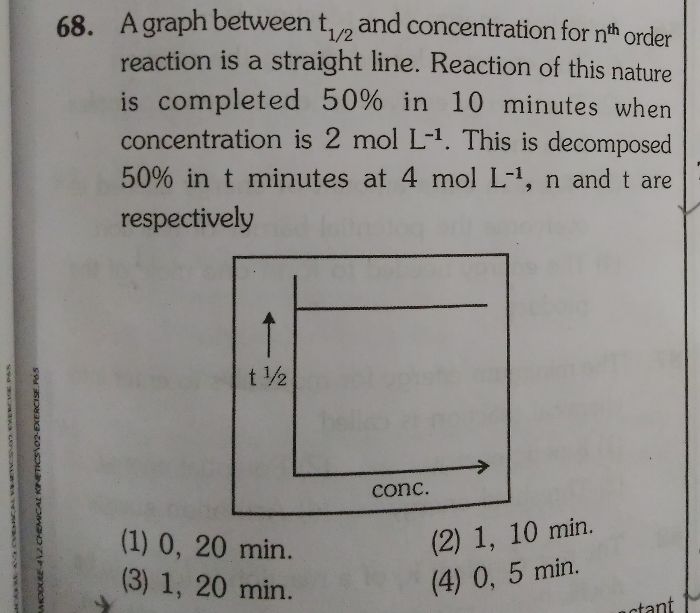
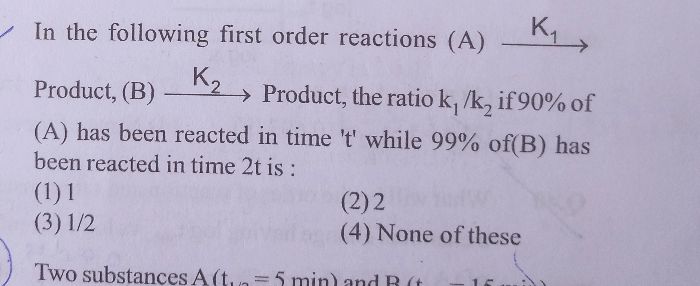CBSE Class 12-science Answered
The half-life is a timescale in which each half-life represents the reduction of the initial population to 50% of its original state. We can represent the relationship by the following equation.

Using the integrated form of the rate law, we can develop a relationship between zero-order reactions and the half-life.

Substitute

Solve for time, 

Rate law for first order reaction:

 is the reaction rate and
is the reaction rate and  is the reaction rate coefficient. In this example, the units of
is the reaction rate coefficient. In this example, the units of  are 1/s. The units can vary with other types of reactions.
are 1/s. The units can vary with other types of reactions.
The half-life is a timescale by which the initial population is decreased by half of its orignal value, t1/2.. We can represent the relationship by the following equation.

Using the integrated form of the rate law, we can develop a relationship between first-order reactions and the half-life.

Substitute

Therefore, for first-order reactions, the half-life is independent of the initial concentration of reactant.