CBSE Class 9 Answered
Respected Sir/Madam,
We have the formula 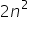 where n is no. of shell which gives us the maximum no. of electrons the shell can accomodate . If an atom has 5 shells then the outermost shell will have 50 electrons according to the formula
where n is no. of shell which gives us the maximum no. of electrons the shell can accomodate . If an atom has 5 shells then the outermost shell will have 50 electrons according to the formula 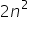 but this contradicts the rule that outermost shell can only have maximum no. of 8 electrons. Please explain...
but this contradicts the rule that outermost shell can only have maximum no. of 8 electrons. Please explain...
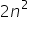 where n is no. of shell which gives us the maximum no. of electrons the shell can accomodate . If an atom has 5 shells then the outermost shell will have 50 electrons according to the formula
where n is no. of shell which gives us the maximum no. of electrons the shell can accomodate . If an atom has 5 shells then the outermost shell will have 50 electrons according to the formula 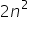 but this contradicts the rule that outermost shell can only have maximum no. of 8 electrons. Please explain...
but this contradicts the rule that outermost shell can only have maximum no. of 8 electrons. Please explain...
Asked by Varsneya Srinivas | 28 Feb, 2015, 11:06: AM
The fifth energy shell has 50 electrons (s, p, d, f, g subshells).
p subshell = 6 electrons
d subshell = 10 electrons
f subshell = 14 electrons
g subshell = 18 electrons
Hence total 50 electrons in the fifth energy level, which confrms 2n2 formula.
Answered by Prachi Sawant | 01 Mar, 2015, 07:55: PM
Application Videos
Concept Videos
CBSE 9 - Chemistry
Asked by archanakad | 25 Aug, 2020, 08:59: PM
CBSE 9 - Chemistry
Asked by Jaiwanthsiva | 15 Apr, 2020, 01:51: PM
CBSE 9 - Chemistry
Asked by Jaiwanthsiva | 12 Apr, 2020, 11:47: AM
CBSE 9 - Chemistry
Asked by snaik0856 | 29 Dec, 2019, 09:47: PM
CBSE 9 - Chemistry
Asked by Regiesmalom99 | 18 Oct, 2019, 01:36: PM
CBSE 9 - Chemistry
Asked by Vishalgarg1234 | 25 Nov, 2018, 03:21: PM
CBSE 9 - Chemistry
Asked by hk.sachdev | 12 Oct, 2018, 10:38: PM
CBSE 9 - Chemistry
Asked by sshivali3333 | 19 Sep, 2018, 11:06: AM
CBSE 9 - Chemistry
Asked by Topperlearning User | 13 May, 2014, 12:49: PM
CBSE 9 - Chemistry
Asked by Topperlearning User | 13 May, 2014, 12:50: PM







 . Then i have to first react Carbon with Oxygen then react that compond with calcium. So how will i know the valency of compound formed from reaction of carbon with oxygen. We can know the valency of elements from their atomic number and electronic configuration, but how can i know the valency of compound?
Please help,waiting for your reply,
Thank you.
. Then i have to first react Carbon with Oxygen then react that compond with calcium. So how will i know the valency of compound formed from reaction of carbon with oxygen. We can know the valency of elements from their atomic number and electronic configuration, but how can i know the valency of compound?
Please help,waiting for your reply,
Thank you. 