NEET Class neet Answered
Respected Sir , Help me
This Question Calculate C. F. S. C
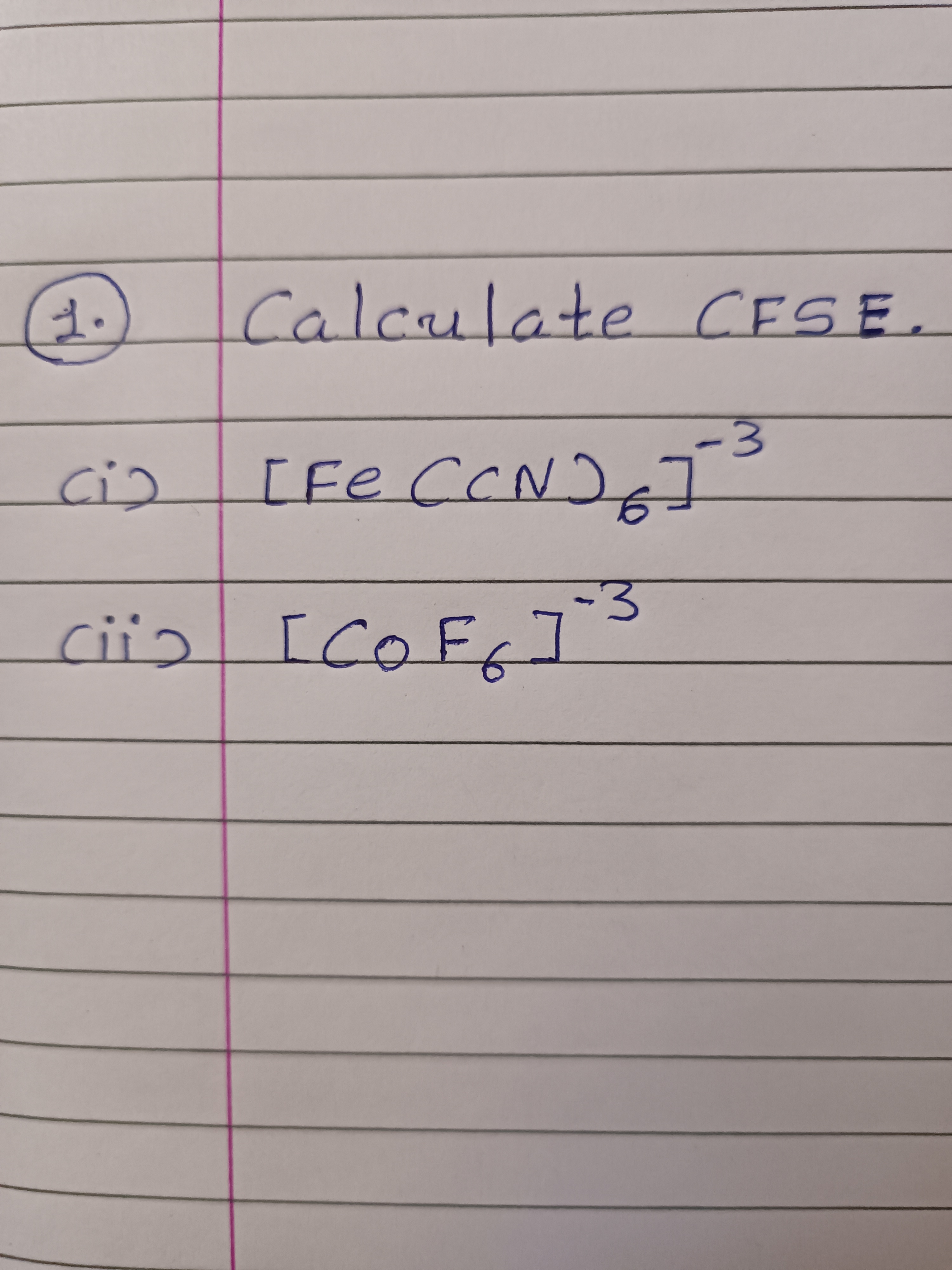
Asked by dipakmodi2309 | 03 Apr, 2023, 09:51: AM
Dear Student,
CFSE Fe(CN)6]3−
It is an octahedral complex.
E.C. of Fe3+ = [Ar]3d5
Cynide is s strong field ligand.
Hence, electron distribution of valence electrons would be t2g = 5 electrons, eg = zero electrons.
∆0 = −0.4 x + 0.6 y
Where, x = no. of electrons in t2g
And y = no. of electrons in eg
∆0 = (−0.4 × 5) + (0.6 × 0) = −2.0
(Kindly ask one query at a time)
Answered by | 03 Apr, 2023, 10:24: AM
Application Videos
Concept Videos
NEET neet - Chemistry
Asked by biswassayan8464 | 21 Apr, 2024, 11:30: AM
NEET neet - Chemistry
Asked by mahendar160786 | 16 Apr, 2024, 09:23: PM
NEET neet - Chemistry
Asked by raomayankup83 | 15 Apr, 2024, 07:46: PM
NEET neet - Chemistry
Asked by muskannawab11 | 14 Apr, 2024, 03:13: PM
NEET neet - Chemistry
Asked by 8239682116rahul | 10 Apr, 2024, 01:48: PM
NEET neet - Chemistry
Asked by tarasingrathod63 | 07 Apr, 2024, 01:07: PM
NEET neet - Chemistry
Asked by fathimahusna6122 | 05 Apr, 2024, 10:25: AM
NEET neet - Chemistry
Asked by vasantagomasi23 | 05 Apr, 2024, 08:35: AM
NEET neet - Chemistry
Asked by ankuruthanuriya | 03 Apr, 2024, 10:56: PM




















