CBSE Class 11-science Answered
Redox Reaction: solve the following equation by ion electron method in acidic medium
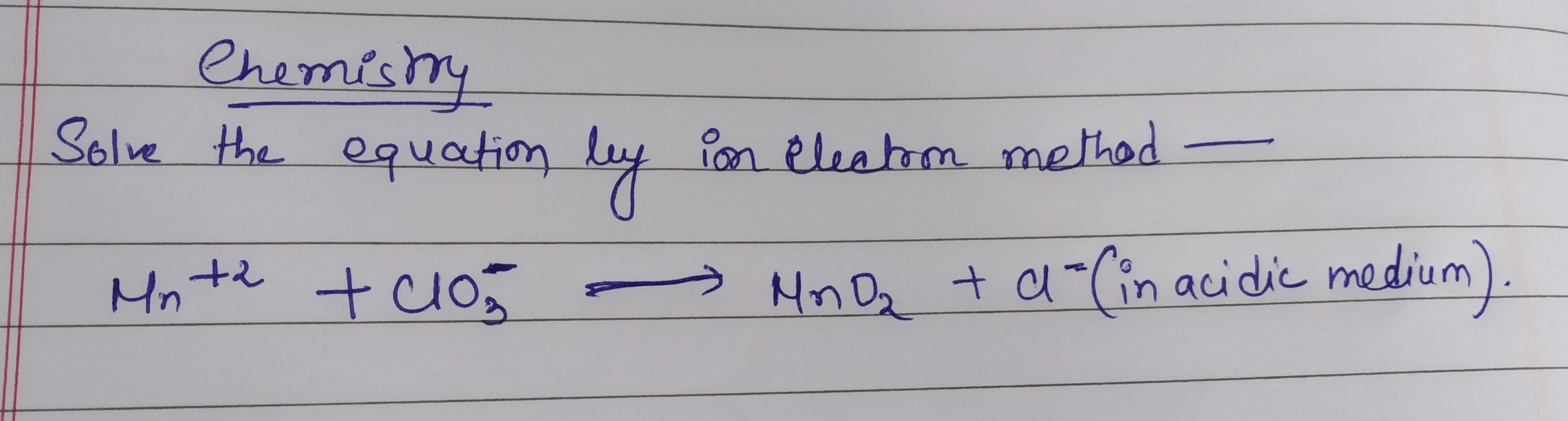
Asked by kkdmmdsd | 09 Jun, 2019, 04:27: PM
In ion electron method, divide the equation in two part
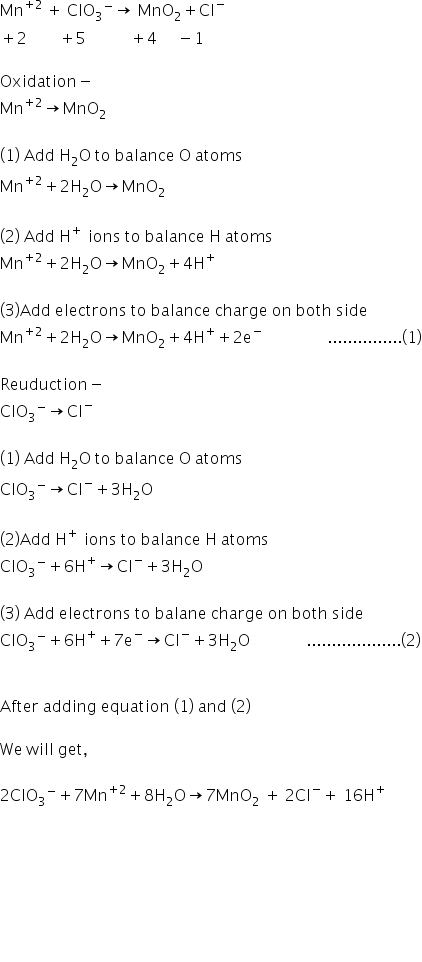
Answered by Ravi | 10 Jun, 2019, 12:43: PM
Concept Videos
CBSE 11-science - Chemistry
Asked by indranibaudya | 03 Oct, 2020, 08:12: PM
CBSE 11-science - Chemistry
Asked by Punshibakhuraijam2015 | 22 Sep, 2019, 08:40: PM
CBSE 11-science - Chemistry
Asked by kkdmmdsd | 09 Jun, 2019, 04:27: PM
CBSE 11-science - Chemistry
Asked by dheerajmathpal374 | 15 Sep, 2018, 10:43: AM
CBSE 11-science - Chemistry
Asked by govtsecschoolnayaganv051 | 19 Aug, 2018, 06:29: PM
CBSE 11-science - Chemistry
Asked by govtsecschoolnayaganv051 | 18 Aug, 2018, 05:08: PM
CBSE 11-science - Chemistry
Asked by govtsecschoolnayaganv051 | 14 Aug, 2018, 06:06: PM
CBSE 11-science - Chemistry
Asked by vaagai2353 | 29 Jun, 2018, 10:29: PM
CBSE 11-science - Chemistry
Asked by g_archanasharma | 22 Mar, 2018, 10:11: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM