CBSE Class 11-science Answered
questions
Asked by adityaduhanomprakash | 22 Aug, 2010, 12:17: AM
It is not possible to answer so many questions together please post them seprately
dsp2 type of hybridization is seen in case of transition metal ions. The orbitals involved in this type of Hybridization are dx2- y2, s and two p. The four dsp2 hybrid orbitals adopt square planar geometry.
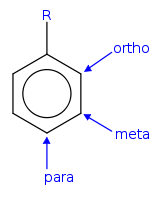
In ortho-substitution, two substituents occupy positions next to each other, which may be numbered 1 and 2.
In meta-substitution the substituents occupy positions 1 and 3
In para-substitution, the substituents occupy the opposite ends i.e 1,4
Answered by | 22 Aug, 2010, 08:58: AM
Concept Videos
CBSE 11-science - Chemistry
Asked by thesouro007 | 20 Mar, 2024, 06:05: AM
CBSE 11-science - Chemistry
Asked by kamalpavenkp123 | 11 Mar, 2024, 02:49: PM
CBSE 11-science - Chemistry
Asked by Trisha Gupta | 30 Oct, 2022, 05:36: PM
CBSE 11-science - Chemistry
Asked by visalvinod06 | 23 Jun, 2022, 07:39: AM
CBSE 11-science - Chemistry
Asked by bhagwatkrutika6 | 22 Jun, 2022, 09:53: PM
CBSE 11-science - Chemistry
Asked by shabnamaijaz83 | 19 Jun, 2022, 10:08: AM
CBSE 11-science - Chemistry
Asked by akankhyapradhan123 | 16 Jan, 2022, 07:46: AM
CBSE 11-science - Chemistry
Asked by abnarsale | 31 Dec, 2021, 10:41: AM
CBSE 11-science - Chemistry
Asked by naveenbahuguna05 | 11 Dec, 2021, 03:19: PM
CBSE 11-science - Chemistry
Asked by akhileshpandeypandey456 | 12 Aug, 2021, 11:09: PM






