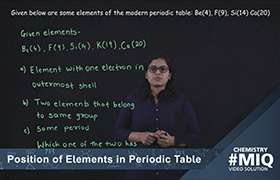
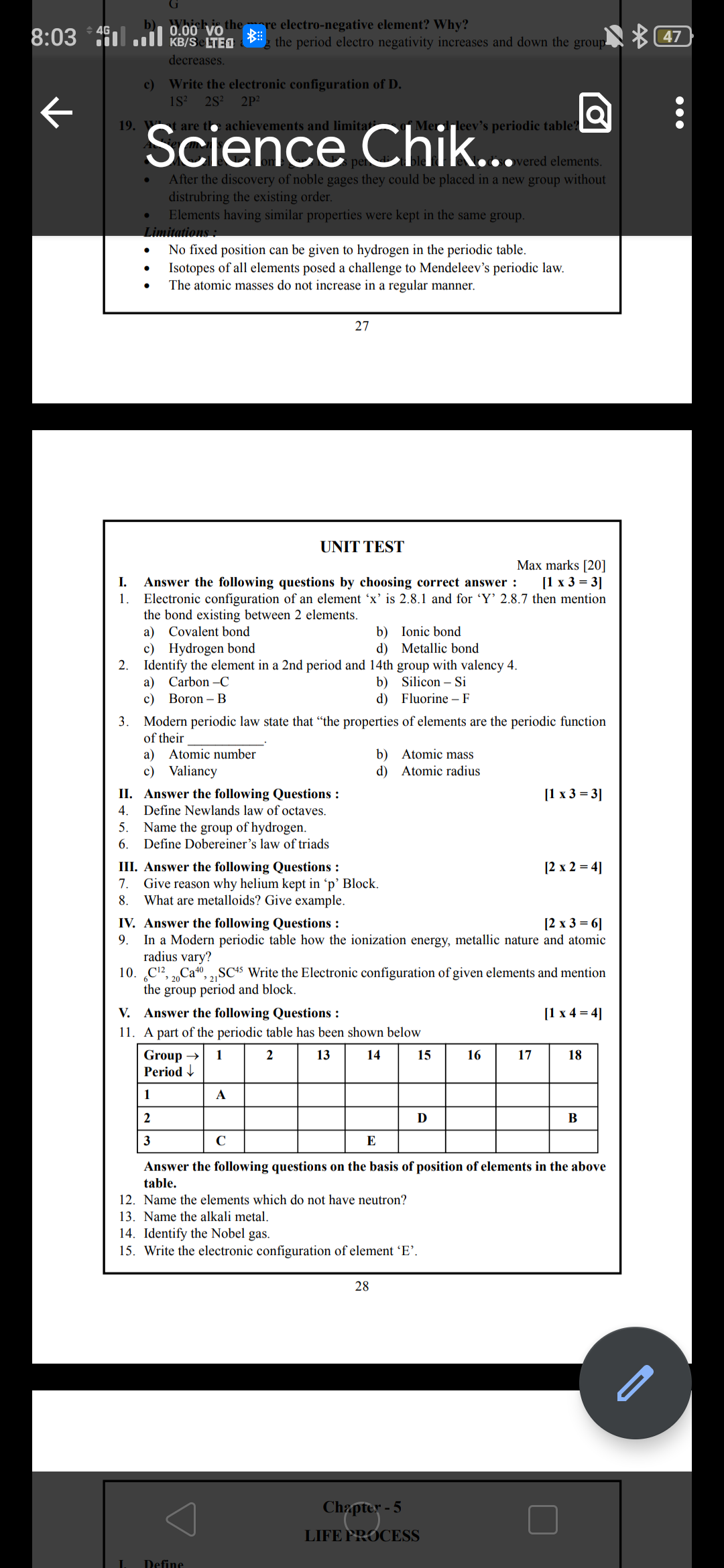
CBSE Class 10 Answered
question
Asked by sweetynam | 18 Feb, 2009, 03:29: PM
Chemical properties of elements are functions of their atomic numbers. The reason is that atomic number decides the number of electrons in valence shell of elements which directly determine the chemical properties of elements.
Elements in mandaleev's periodic table are arranged in increasing order of their masses. Thus as per mandaleev, the chemical properties of elements are functions of their masses. Thus there arrangement is different from those in modern periodic table which has elements arranged as per modern atomc law as stated in the first line.
Answered by | 19 Feb, 2009, 02:00: AM
Application Videos
Concept Videos
CBSE 10 - Chemistry
Asked by dhumalchhaya13 | 26 May, 2022, 11:05: PM
CBSE 10 - Chemistry
Asked by kanchanbalpande82 | 12 Apr, 2022, 11:01: AM
CBSE 10 - Chemistry
Asked by kanchanbalpande82 | 12 Apr, 2022, 11:01: AM
CBSE 10 - Chemistry
Asked by waghmaresheetal78 | 27 Dec, 2021, 05:02: PM
CBSE 10 - Chemistry
Asked by ksheera36 | 03 Jun, 2021, 08:35: PM
CBSE 10 - Chemistry
Asked by vungtsaniyanthan | 16 May, 2021, 06:32: PM
CBSE 10 - Chemistry
Asked by sinhagopalakumara | 01 May, 2021, 08:16: PM
CBSE 10 - Chemistry
Asked by advssdrall | 26 Mar, 2021, 07:43: AM
CBSE 10 - Chemistry
Asked by kavithasenthil148 | 06 Feb, 2021, 07:09: PM
CBSE 10 - Chemistry
Asked by mina6poonam | 21 Jan, 2021, 04:57: PM