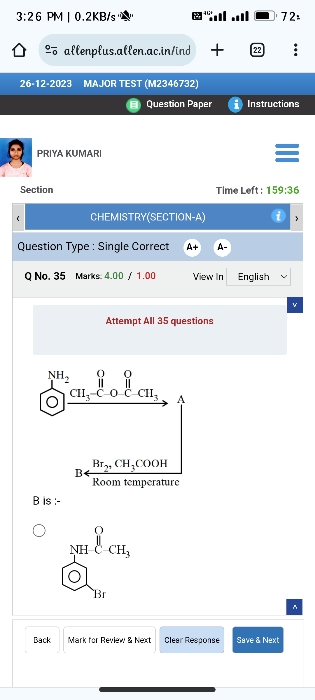
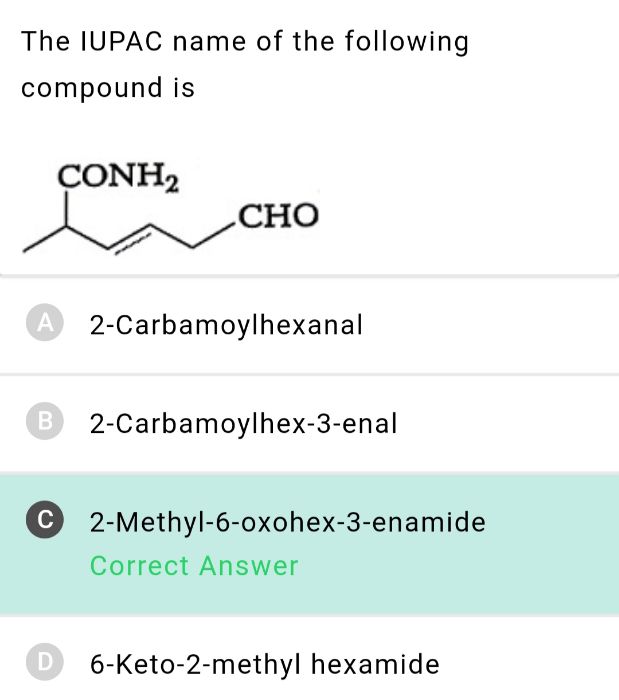
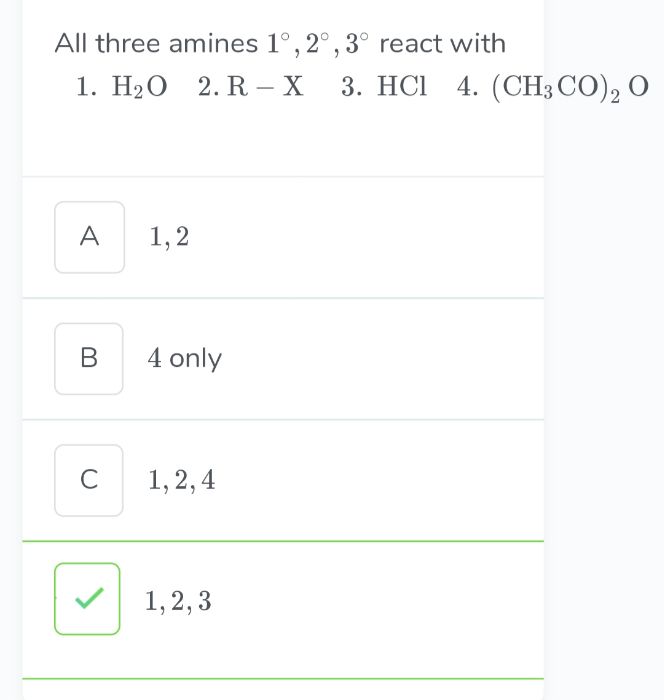
CBSE Class 12-science Answered
The correct order of boiling points is:
C3H8, CH3CH2NH2, C2H5OH, HCOOH
Amines have lower boiling pout than those of alcohols or carboxylic acids because O-H bond present in alcohols and carboxylic acids are more polar than the N-H bond in amines and hence the hydrogen bonds in alcohols and carboxylic acids are strong and therefore they have higher boiling points.
Acids have high boiling point than alcohols because as compared to alcohols, the O-H bond in acids is more strongly polarized due to the presence of adjacent electron withdrawing carbonyl group and hence they can form stronger hydrogen bonds and also the molecules of carboxylic acids are held together by two hydrogen bonds and therefore form cyclic dimmers.