CBSE Class 11-science Answered
Q) 

Asked by araima2001 | 29 Mar, 2017, 06:21: AM
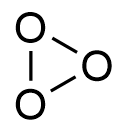
A = H3BO3
B = B(OC2H5)3
Ethyl alcohol on heating with boric acid gives a salt of ethyl borate which burns with a green edged flame.
Answered by Vaibhav Chavan | 29 Mar, 2017, 11:50: AM
Concept Videos
CBSE 11-science - Chemistry
Asked by ABHILASHA | 08 Sep, 2019, 06:46: PM
CBSE 11-science - Chemistry
Asked by vishakhachandan026 | 12 Jun, 2019, 09:20: AM
CBSE 11-science - Chemistry
Asked by pb_ckt | 28 Apr, 2019, 01:40: PM
CBSE 11-science - Chemistry
Asked by satya785583 | 16 Mar, 2019, 09:18: PM
CBSE 11-science - Chemistry
Asked by govtsecschoolnayaganv051 | 03 Jan, 2019, 01:09: PM
CBSE 11-science - Chemistry
Asked by govtsecschoolnayaganv051 | 03 Jan, 2019, 12:34: PM
CBSE 11-science - Chemistry
Asked by anushkamoudgil123 | 02 Nov, 2018, 05:47: AM
CBSE 11-science - Chemistry
Asked by arunavamitra50 | 04 Jul, 2018, 09:11: PM
CBSE 11-science - Chemistry
Asked by arunavamitra50 | 04 Jul, 2018, 09:10: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 31 May, 2016, 01:10: PM