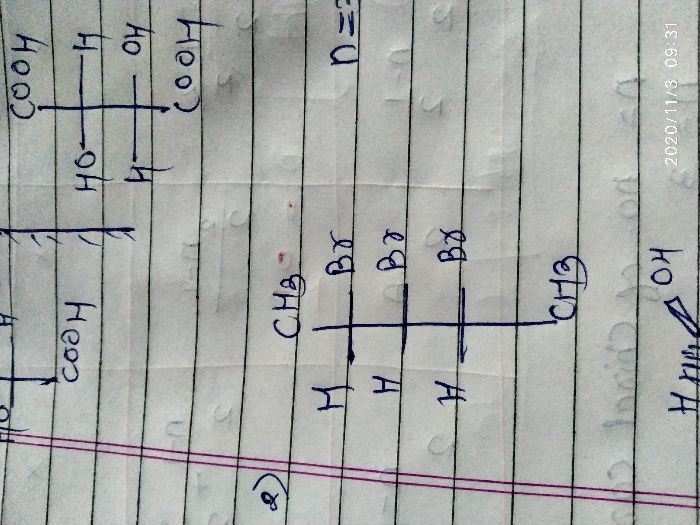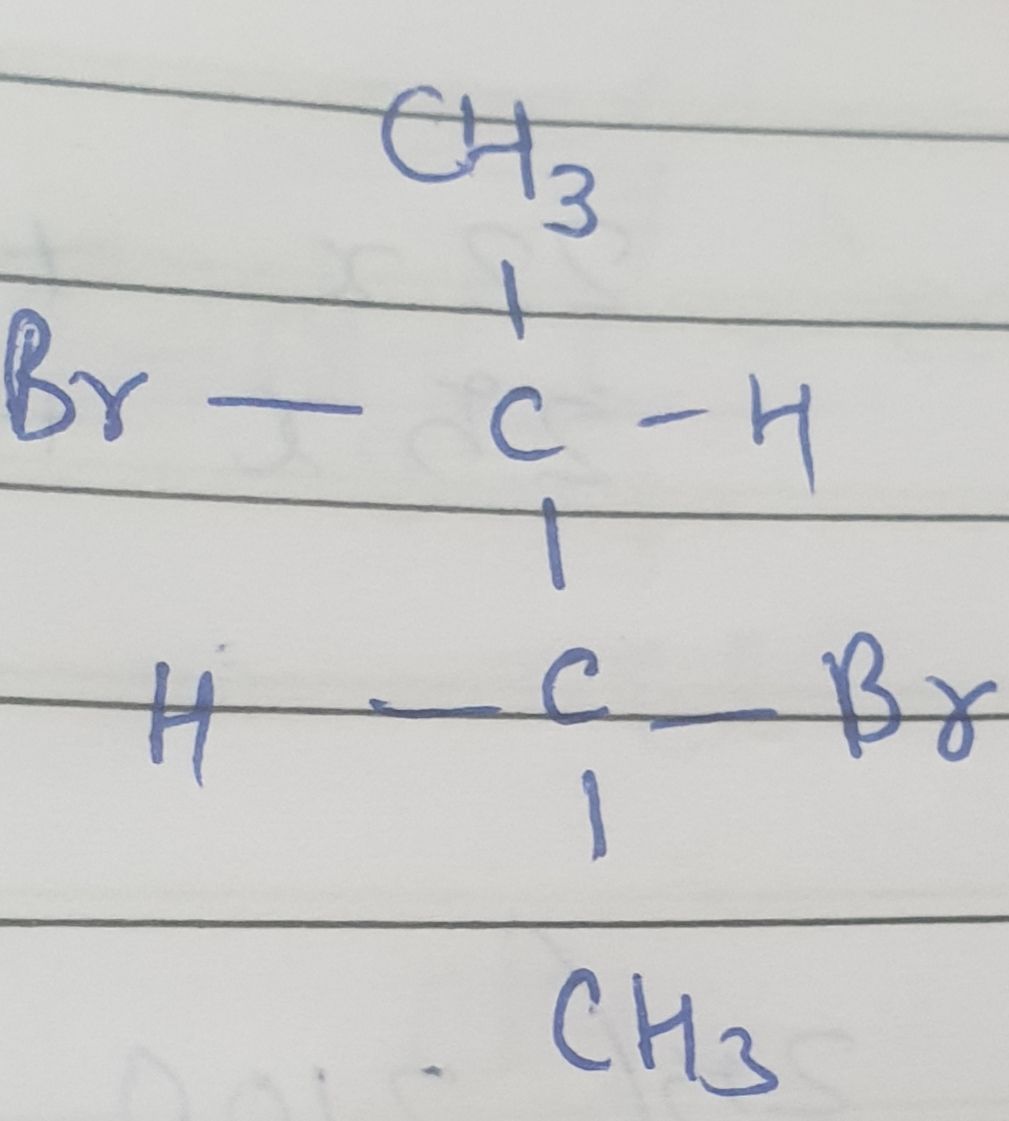CBSE Class 11-science Answered
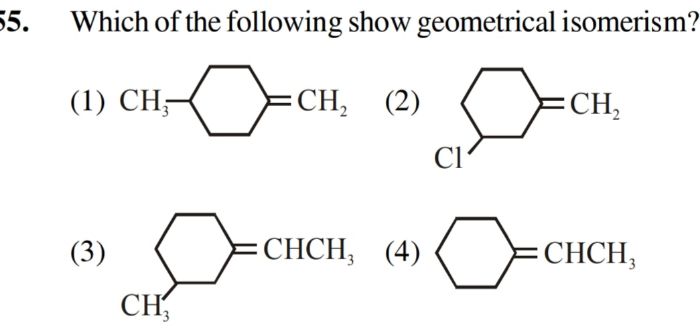
Q)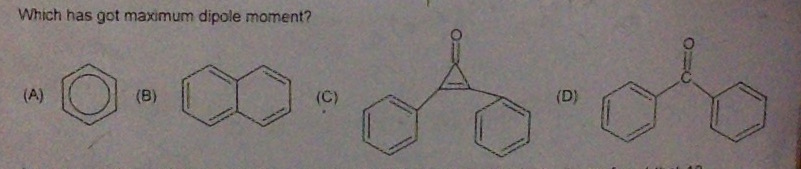

Asked by araima2001 | 07 Apr, 2017, 04:54: AM
The correct option is C.
Benzene and naphthalene, both have zero dipole moment.
Dipole moment of benzophenone is 2.98 D.
Dipole moment of 2,3-diphenylcycloprop-2-enone is maximum that is 5.08 D.
Answered by Prachi Sawant | 07 Apr, 2017, 12:41: PM
Concept Videos
CBSE 11-science - Chemistry
Asked by ap4450962 | 12 Mar, 2024, 07:35: PM
CBSE 11-science - Chemistry
Asked by shahintkjnv2016 | 13 Jun, 2022, 07:17: PM
CBSE 11-science - Chemistry
Asked by rohus442 | 03 Nov, 2020, 09:31: AM
CBSE 11-science - Chemistry
Asked by ashok.amireddi | 01 May, 2020, 10:33: PM
CBSE 11-science - Chemistry
Asked by mufeedatvp2000 | 13 Apr, 2020, 08:36: PM
CBSE 11-science - Chemistry
Asked by prakriti12oct | 12 Dec, 2019, 12:00: AM
CBSE 11-science - Chemistry
Asked by ajaysankhala051 | 02 Sep, 2019, 02:26: PM
CBSE 11-science - Chemistry
Asked by musira29rahman | 30 Aug, 2019, 05:09: PM
CBSE 11-science - Chemistry
Asked by govtsecschoolnayaganv051 | 27 Aug, 2019, 06:33: PM
CBSE 11-science - Chemistry
Asked by ajaysankhala051 | 26 Aug, 2019, 09:39: PM