CBSE Class 11-science Answered
Q.
Asked by ritamkumar | 16 Mar, 2010, 06:13: AM
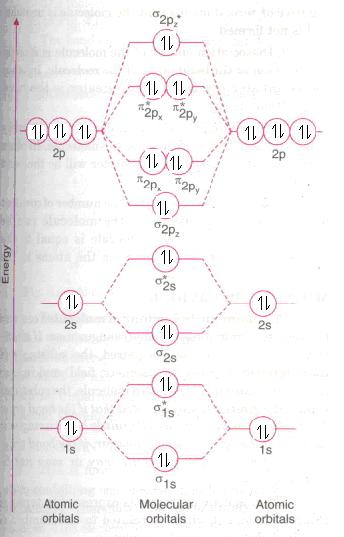
Bond order=1/2(Number of electrons in bonding molecular orbital-Number of electrons in antibonding molecular orbital)
=1/2(20-20)=0
Since bond order is 0 it suggest that neon molecule being unstable does not exist
Answered by | 16 Mar, 2010, 09:05: AM
Concept Videos
CBSE 11-science - Chemistry
Asked by thesouro007 | 20 Mar, 2024, 06:05: AM
CBSE 11-science - Chemistry
Asked by kamalpavenkp123 | 11 Mar, 2024, 02:49: PM
CBSE 11-science - Chemistry
Asked by Trisha Gupta | 30 Oct, 2022, 05:36: PM
CBSE 11-science - Chemistry
Asked by visalvinod06 | 23 Jun, 2022, 07:39: AM
CBSE 11-science - Chemistry
Asked by bhagwatkrutika6 | 22 Jun, 2022, 09:53: PM
CBSE 11-science - Chemistry
Asked by shabnamaijaz83 | 19 Jun, 2022, 10:08: AM
CBSE 11-science - Chemistry
Asked by akankhyapradhan123 | 16 Jan, 2022, 07:46: AM
CBSE 11-science - Chemistry
Asked by abnarsale | 31 Dec, 2021, 10:41: AM
CBSE 11-science - Chemistry
Asked by naveenbahuguna05 | 11 Dec, 2021, 03:19: PM
CBSE 11-science - Chemistry
Asked by akhileshpandeypandey456 | 12 Aug, 2021, 11:09: PM