CBSE Class 12-science Answered
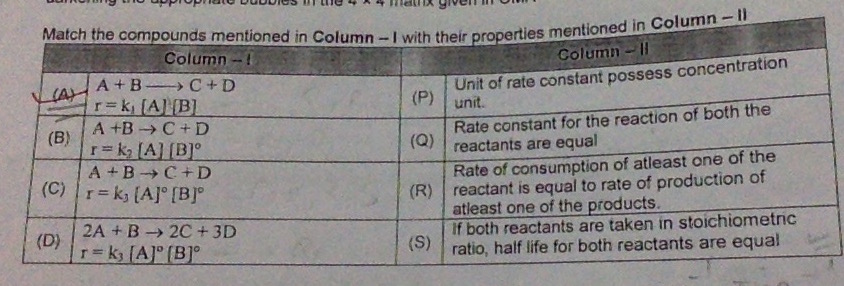
Q) what will the correct match of (B) & also explain the reason (one or more than one correct )
Asked by araima2001 | 26 Dec, 2016, 08:48: PM
Dear araima2001@yahoo.com
Thanks for asking us a question in Ask the Expert section of TopperLearning.com.
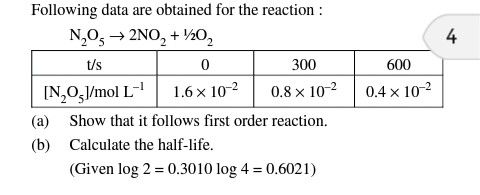
1. Unit of rate constant possess concentration unit: This statement is true for zero order, 2nd order and 3rd order not for 1st order.
2. Rate constant for the reaction of both the reactants are equal : This statement is true only for zero order reaction.
3. Rate of consumption of at least one of the reactants is equal to the rate of production of at least one of the products: This statement is true only for 1st order reaction.
4. If both reactants are taken in stoichiometric ratio, half life for both reactants are equal: This is true for only 2nd order reaction.
From above observationscorrect match for (B) will be Rate of consumption of at least one of the reactants is equal to the rate of production of at least one of the products.
Regards
Topperlearning Team.
Answered by Arvind Diwale | 27 Dec, 2016, 03:48: PM
Concept Videos
CBSE 12-science - Chemistry
Asked by rchaitra1204 | 07 Sep, 2020, 09:43: AM
CBSE 12-science - Chemistry
Asked by arunhys123 | 04 Jul, 2020, 07:36: PM
CBSE 12-science - Chemistry
Asked by dhruvrana348 | 28 Jun, 2020, 08:58: AM
CBSE 12-science - Chemistry
Asked by lovemaan5500 | 06 Jan, 2020, 03:39: PM
CBSE 12-science - Chemistry
Asked by lovemaan5500 | 18 Sep, 2019, 10:02: PM
CBSE 12-science - Chemistry
Asked by sanjeet.kumar | 12 Mar, 2019, 02:20: PM
CBSE 12-science - Chemistry
Asked by manpreetkaur19971993 | 10 Jan, 2019, 07:06: AM
CBSE 12-science - Chemistry
Asked by rohitraman1115 | 22 Jul, 2018, 08:27: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 27 Mar, 2014, 01:37: PM