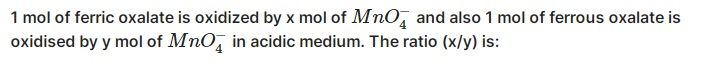CBSE Class 11-science Answered
Q] What is the difference between mass number & atomic mass ?
Asked by Prahnesh | 25 Jun, 2016, 07:05: PM
"Mass number" is simply the total number of nuclear particles (protons plus neutrons).
"Atomic mass" is the actual mass of the atom.
Remember that protons and neutrons do not have masses of exactly one amu.
Even if they did, the mass of the nucleus is not the sum of the masses of the nuclear particles.
There is only one isotope of one element that is carbon for which the mass number and atomic mass are exactly the same. That is carbon-12. It is because the amu is defined as 1/12 of the mass of a carbon-12 atom.
Answered by Prachi Sawant | 27 Jun, 2016, 11:30: AM
Concept Videos
CBSE 11-science - Chemistry
Asked by jayag1298 | 08 Apr, 2024, 03:14: PM
CBSE 11-science - Chemistry
Asked by omniscientnjf2021 | 07 Apr, 2024, 10:18: PM
CBSE 11-science - Chemistry
Asked by hcnainwal | 15 Jun, 2023, 10:39: AM
CBSE 11-science - Chemistry
Asked by Jprmumal29 | 18 Dec, 2022, 09:48: PM
CBSE 11-science - Chemistry
Asked by mallikarjunasangi28 | 22 Jul, 2022, 07:57: PM
CBSE 11-science - Chemistry
Asked by vedwatisharma79 | 10 Jun, 2022, 05:27: PM
CBSE 11-science - Chemistry
Asked by thathvakunjusree | 10 Dec, 2021, 06:46: AM
CBSE 11-science - Chemistry
Asked by udheshraddha2004 | 28 Oct, 2021, 09:37: PM
CBSE 11-science - Chemistry
Asked by arunparewa2000 | 27 Oct, 2021, 06:59: PM
CBSE 11-science - Chemistry
Asked by arttameher038 | 23 Aug, 2021, 07:06: AM