CBSE Class 9 Answered
Q the half line of a radioactive isotope is 1.5 hrs. The mass of it that remains undecayed after 6 hours if the initial mass of spectrum of the isotope of 32g is
Asked by vikasg13.hardware | 15 Nov, 2017, 01:36: PM
Given:
half-life (T) = 1.5 hrs
initial mass of the isotope (A0) = 32 gms
Time = 6 hrs
Find: final mass of isotope ?
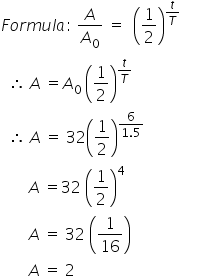
the mass of that remains undecayed after 6 hours willbe 2 gms
Answered by Ramandeep | 15 Nov, 2017, 02:50: PM
Application Videos
Concept Videos
CBSE 9 - Chemistry
Asked by namyairmalwar9803.5sdatl | 07 Jun, 2020, 01:47: PM
CBSE 9 - Chemistry
Asked by pradoshsanesar | 10 Jan, 2020, 12:01: PM
CBSE 9 - Chemistry
Asked by maltidevi8800 | 06 Dec, 2019, 09:29: PM
CBSE 9 - Chemistry
Asked by ABHILASHA | 22 Aug, 2019, 08:46: AM
CBSE 9 - Chemistry
Asked by dubeykrishna49025 | 14 Oct, 2018, 10:02: PM
CBSE 9 - Chemistry
Asked by Topperlearning User | 13 May, 2014, 03:03: PM
CBSE 9 - Chemistry
Asked by Topperlearning User | 13 May, 2014, 03:03: PM
CBSE 9 - Chemistry
Asked by Topperlearning User | 13 May, 2014, 03:04: PM
CBSE 9 - Chemistry
Asked by Topperlearning User | 13 May, 2014, 03:04: PM
CBSE 9 - Chemistry
Asked by Topperlearning User | 13 May, 2014, 03:05: PM









