CBSE Class 11-science Answered
Q) i am a liitle confused between (I)&(II) whose basic strength will be more & why??? plz... kindly clarify this doubt...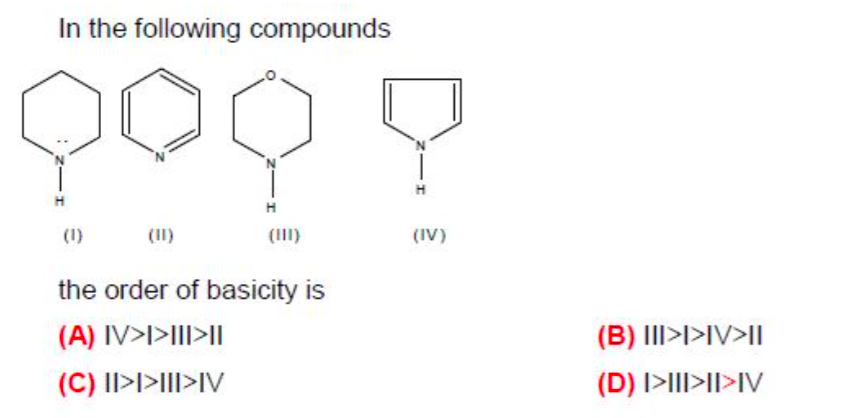

Asked by araima2001 | 21 Apr, 2017, 09:09: PM
Structure (I) is more basic than structure (II) as the lone pair of electrons on nitrogen in structure (II) are involved in the aromaticity of the ring or say they are swimming in the ring and not readily availabale for donation while the lone pair of elecrtons on nitrogen in structure (I) are readily available for donation.
More the electron donating property of the molecule, more is the basicity.
Answered by Prachi Sawant | 23 Apr, 2017, 07:52: PM
Concept Videos
CBSE 11-science - Chemistry
Asked by neet2025targetgo | 25 Mar, 2024, 10:13: AM
CBSE 11-science - Chemistry
Asked by pamjat.8888 | 31 Jan, 2024, 11:31: AM
CBSE 11-science - Chemistry
Asked by sahumahesh3973 | 20 Jan, 2024, 06:33: PM
CBSE 11-science - Chemistry
Asked by aswintj2007 | 07 Jan, 2024, 08:53: PM
CBSE 11-science - Chemistry
Asked by dipalisingh0908 | 05 Nov, 2023, 02:24: PM
CBSE 11-science - Chemistry
Asked by badalbehera258369 | 19 Oct, 2023, 02:01: PM
CBSE 11-science - Chemistry
Asked by prakrutikhosla | 16 Sep, 2023, 06:31: PM
CBSE 11-science - Chemistry
Asked by hitanshu04 | 17 Jun, 2021, 07:20: PM
CBSE 11-science - Chemistry
Asked by ABHILASHA | 22 Aug, 2020, 05:13: AM
CBSE 11-science - Chemistry
Asked by ABHILASHA | 22 Aug, 2020, 05:09: AM








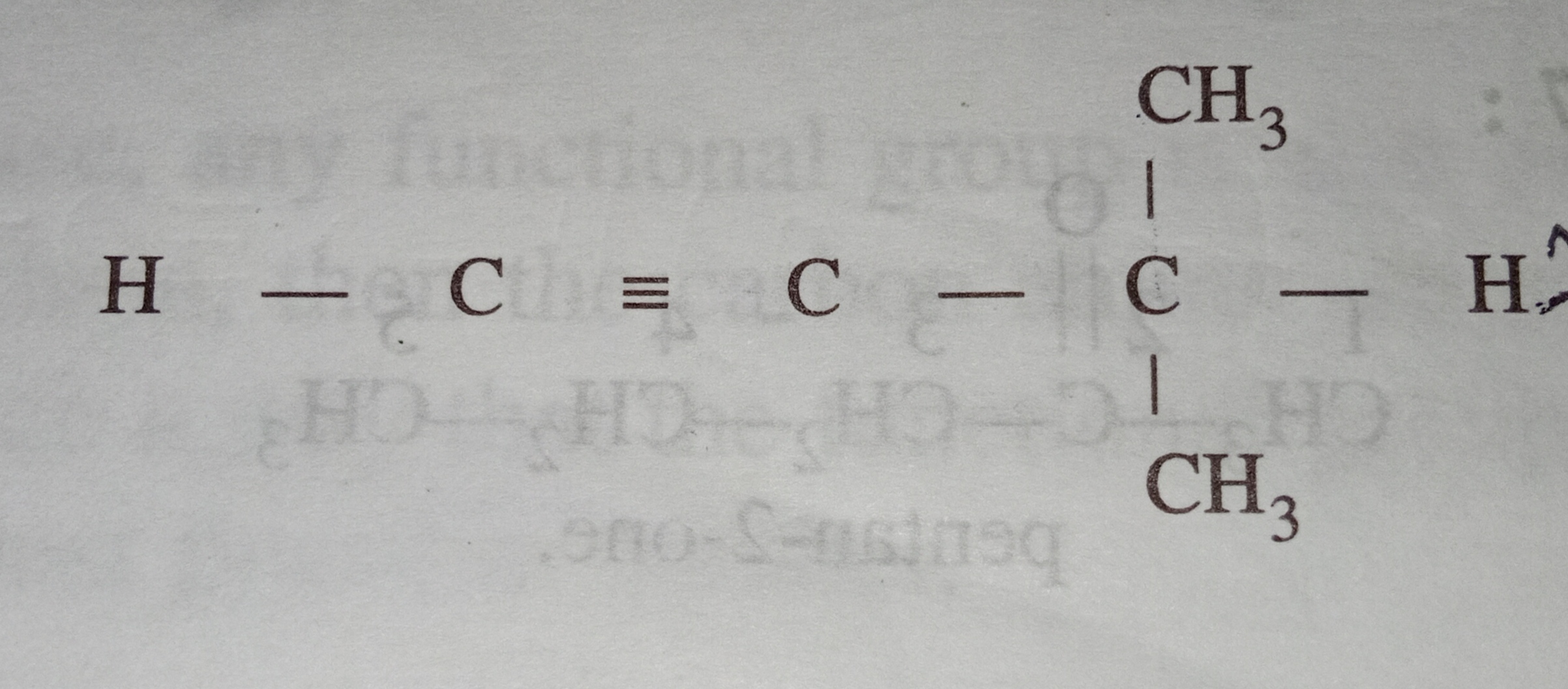
 hybridised ,how will it be classified?
hybridised ,how will it be classified?