CBSE Class 11-science Answered
Pls solve it.
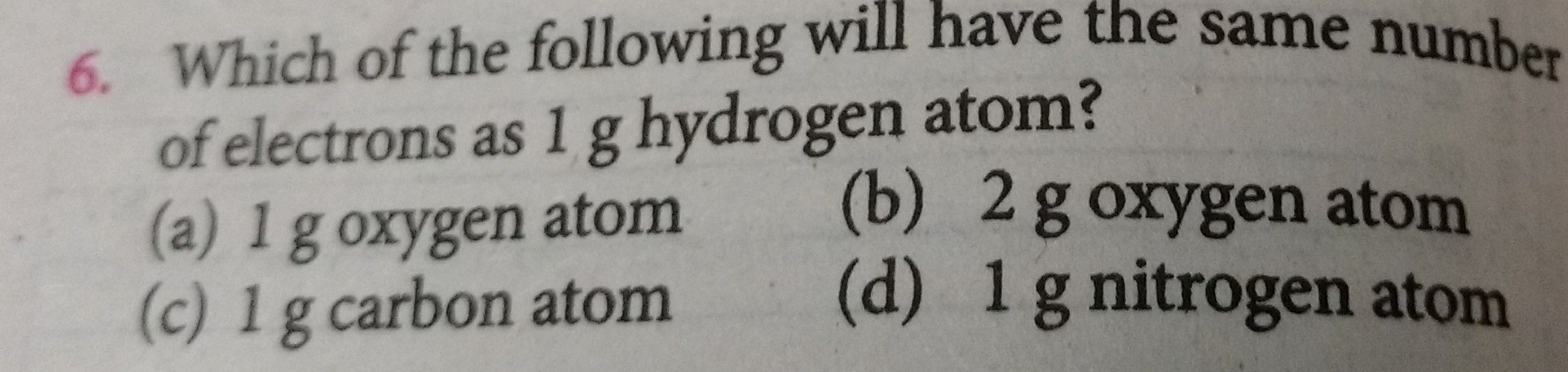
Asked by pb_ckt | 01 May, 2019, 10:43: PM
Option (b) is correct.
Given:
1 gm of hydrogen atoms
We know,
1 gm of hydrogen atoms = 1 mole
1 mole of hydrogen =6.022×1023 atoms
1 atom of hydrogen conatains 1 electron
Therefore 1 gm hydrogen atoms contains 6.022×1023 electrons.
Now, for 1 gm of oxygen atoms
No. of moles of oxygen
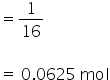
1 mol of oxygen atoms = 6.022×1023 atoms
so 0.0625 mol f oxygen atoms =
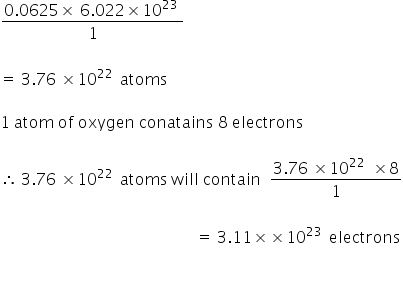
For 2 gm oxygen atoms
No. of moles of oxygen
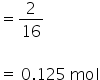
1 mol of oxygen atoms = 6.022×1023 atoms
so 0.125 mol f oxygen atoms =
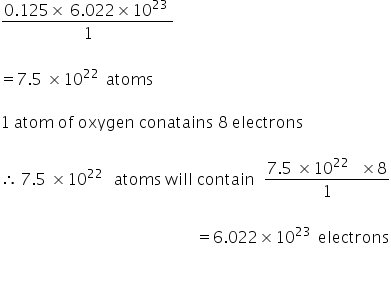
Therefore 2 gm of oxygen atoms will conatain 6.022×1023 electrons which are same as in 1 gm hydrogen atom.
Answered by Varsha | 02 May, 2019, 11:58: AM
Concept Videos
CBSE 11-science - Chemistry
Asked by manikandanragul1 | 11 Apr, 2024, 09:02: AM
CBSE 11-science - Chemistry
Asked by nikhithaguguloth14 | 29 Mar, 2024, 08:15: PM
CBSE 11-science - Chemistry
Asked by sumedhasingh238 | 27 Mar, 2024, 11:04: PM
CBSE 11-science - Chemistry
Asked by avijotsingh946431 | 22 Feb, 2024, 05:36: PM
CBSE 11-science - Chemistry
Asked by gurmelsinghray | 21 Feb, 2024, 08:43: AM
CBSE 11-science - Chemistry
Asked by bablipanwar893 | 01 Jul, 2023, 12:25: PM
CBSE 11-science - Chemistry
Asked by saijagdale9 | 19 Jun, 2023, 02:34: PM
CBSE 11-science - Chemistry
Asked by kdimple765 | 17 Jul, 2022, 01:24: PM
CBSE 11-science - Chemistry
Asked by alfirozislam900 | 03 Jul, 2022, 01:24: PM
CBSE 11-science - Chemistry
Asked by alfirozislam900 | 03 Jul, 2022, 01:23: PM




