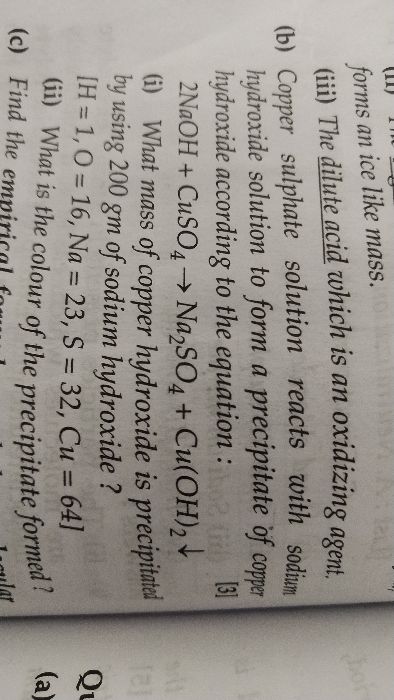ICSE Class 10 Answered
Pls solve it.

Asked by prodyumna02 | 20 Mar, 2019, 04:15: PM
We know that hydrocarbon contains only hydrogen and carbon.
(a) Therefore hydrocarbon X contains hydrogen and carbon.
(b) The copper oxide acts as an oxidising agent.
(c)
(i) No. of moles of CO2
The volume of CO2 = 224 ml
We know,
1 mole = 22400 ml
So 224 ml will be
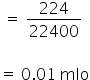
Also,
(ii) 22400 ml contains 12 gm of carbon
That is 1 mole contains 12 g of C
So 0.01 mole will contain = 0.12 g of C
(iii) Mass of hydrogen = total mass of hydrocarbon − mass of carbon
mass of hydrocarbon = 0.145 g
Mass of hydrogen = 0.145 − 0.12
= 0.025 g
(iv) Mass of carbon = 0.12 gm
Mass of hydrogen = 0.025 g
Atomic mass of carbon = 12
Atomic mass of hydrogen = 1
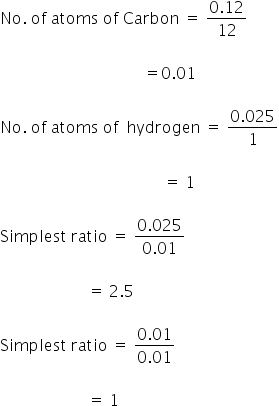
Multiply 2.5 and 1 by 2 to get the simplest ratio,
So, the empirical formula is C2H5
Answered by Varsha | 20 Mar, 2019, 07:20: PM
Application Videos
Concept Videos
ICSE 10 - Chemistry
Asked by jrvedant208 | 05 Feb, 2024, 10:37: PM
ICSE 10 - Chemistry
Asked by rashikulkarni28 | 10 Jul, 2022, 10:13: PM
ICSE 10 - Chemistry
Asked by rashikulkarni28 | 25 Jun, 2022, 10:24: PM
ICSE 10 - Chemistry
Asked by palshivom72 | 14 Jul, 2020, 07:56: PM
ICSE 10 - Chemistry
Asked by jhabijay01 | 27 May, 2020, 12:20: PM
ICSE 10 - Chemistry
Asked by aashimegh | 04 Sep, 2019, 08:53: AM
ICSE 10 - Chemistry
Asked by aashimegh | 04 Sep, 2019, 08:37: AM
ICSE 10 - Chemistry
Asked by aashimegh | 28 Aug, 2019, 05:25: PM