CBSE Class 12-science Answered
pls explain
Asked by mufeedatvp2000 | 18 Apr, 2020, 02:20: PM
Question is posted with error . transistion should be n =2 to n= 1 state.
Energy State with quantum number n = 2 is excited state and other one with quantum number n =1 is ground state
---------------------------------------
if v1 and v2 are orbital velocities of ground state and excited state, then they are related as v2 = (1/2) v1
orbital velocity of ground state, v1 = 2.19 × 106 m/s
Hence orbital velocity of excited state, v2 = (1/2) × 2.19 × 106 = 1.095 × 106 m/s
Orbital radius of ground state and excited state are related as,
r2 = 4 r1 = 4 × 5.29 × 10-11 m = 2.116 × 10-10 m
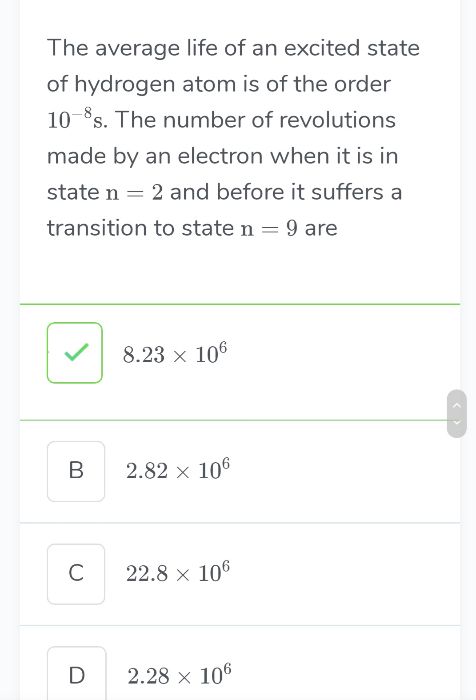
if electron stays 10-8 seconds in excited state and it makes n number of revolutions then we have
( n × 2π r2 ) / v2 = 10-8
Hence number of revolution, n = [ 10-8 × 1.095 × 106 ] / [ 2π × 2.116 × 10-10 ] = 8.236 × 106
Answered by Thiyagarajan K | 19 Apr, 2020, 08:59: PM
Concept Videos
CBSE 12-science - Physics
Asked by dasrituparna1999 | 13 Apr, 2024, 06:56: AM
CBSE 12-science - Physics
Asked by dasrituparna1999 | 12 Apr, 2024, 09:26: PM
CBSE 12-science - Physics
Asked by mishrigupta19319 | 08 Apr, 2024, 06:28: PM
CBSE 12-science - Physics
Asked by madhav9119887644 | 07 Apr, 2024, 08:10: PM
CBSE 12-science - Physics
Asked by mishrigupta19319 | 07 Apr, 2024, 11:23: AM
CBSE 12-science - Physics
Asked by adityagalar2007 | 06 Apr, 2024, 01:06: PM
CBSE 12-science - Physics
Asked by amlanmcob | 06 Apr, 2024, 12:27: PM
CBSE 12-science - Physics
Asked by hussain221man | 05 Apr, 2024, 08:44: PM
CBSE 12-science - Physics
Asked by mishrigupta19319 | 05 Apr, 2024, 12:01: PM
CBSE 12-science - Physics
Asked by manishamunda787 | 02 Apr, 2024, 11:07: AM