CBSE Class 10 Answered
Redox Reaction:
A chemical reaction in which loss of electrons and gain of electrons occur simultaneously is called a redox reaction.
Example:
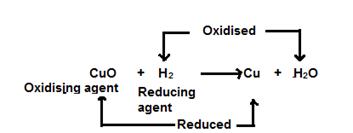
In this reaction, hydrogen acts as a reducing agent and reduces copper oxide to copper. This is a reduction reaction.
Reduction: Cu+2 + 2e−→ Cu
Simultaneously, copper oxide acts as an oxidising agent and oxidises hydrogen to water. This is an oxidation reaction.
Oxidation: 2H - 2e− → 2H+
Following are some examples:The O.N. of S increases from -2 to 0. So it is undergoing oxidation and O.N. of Cl2 decreases from 0 to -1. So it is undergoing reduction. Therefore it is a redox reaction.
CuO + H2 → Cu +H2O
In this redox reaction, CuO is getting reduced to Cu since Oxygen is getting removed. So, the conversion of CuO to Cu is reduction reaction. H2 is getting oxidised to H2O. So, it is oxidation reaction.
Fe(s) + CuSO4(aq) → FeSO4(aq) + Cu(s)
Oxidation state of Fe changes from 0 to +2 and oxidation state of Cu changes from +2 to 0.
MnO4-+ I- → MnO2 + I2
Here I- is oxidised to I2 and MnO4 - is reduced to MnO2.
Pb + PbO2 + 2H2SO4 → 2PbSO4 + 2H2O
In this reaction, Pb is getting oxidised to PbSO4 and PbO2 ias reduced to PbSO4.









