CBSE Class 11-science Answered
Please write the name of the compound and explain the steps involved in writing the name.
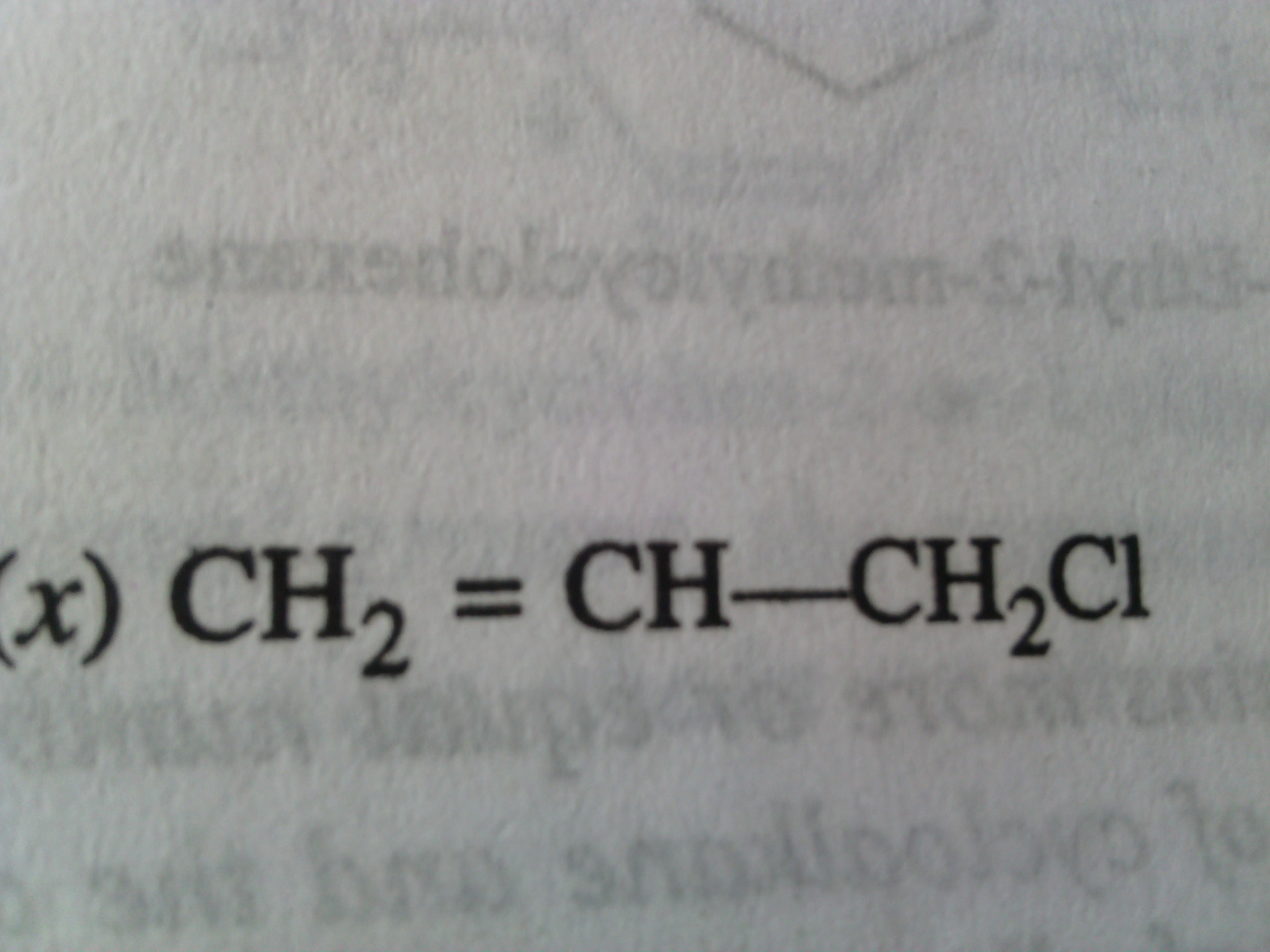
Asked by Varsneya Srinivas | 11 Jan, 2017, 10:52: AM
Parent chain has 3 carbon atoms.
The numbering will start from the double bond so that it gets least numbering.
Then we have –Cl attached to the third carbon atom.
The IUPAC name of the compound is 3-chloroprop-1-ene.
Answered by Vaibhav Chavan | 11 Jan, 2017, 06:20: PM
Concept Videos
CBSE 11-science - Chemistry
Asked by neet2025targetgo | 25 Mar, 2024, 10:13: AM
CBSE 11-science - Chemistry
Asked by pamjat.8888 | 31 Jan, 2024, 11:31: AM
CBSE 11-science - Chemistry
Asked by sahumahesh3973 | 20 Jan, 2024, 06:33: PM
CBSE 11-science - Chemistry
Asked by aswintj2007 | 07 Jan, 2024, 08:53: PM
CBSE 11-science - Chemistry
Asked by dipalisingh0908 | 05 Nov, 2023, 02:24: PM
CBSE 11-science - Chemistry
Asked by badalbehera258369 | 19 Oct, 2023, 02:01: PM
CBSE 11-science - Chemistry
Asked by prakrutikhosla | 16 Sep, 2023, 06:31: PM
CBSE 11-science - Chemistry
Asked by hitanshu04 | 17 Jun, 2021, 07:20: PM
CBSE 11-science - Chemistry
Asked by ABHILASHA | 22 Aug, 2020, 05:13: AM
CBSE 11-science - Chemistry
Asked by ABHILASHA | 22 Aug, 2020, 05:09: AM








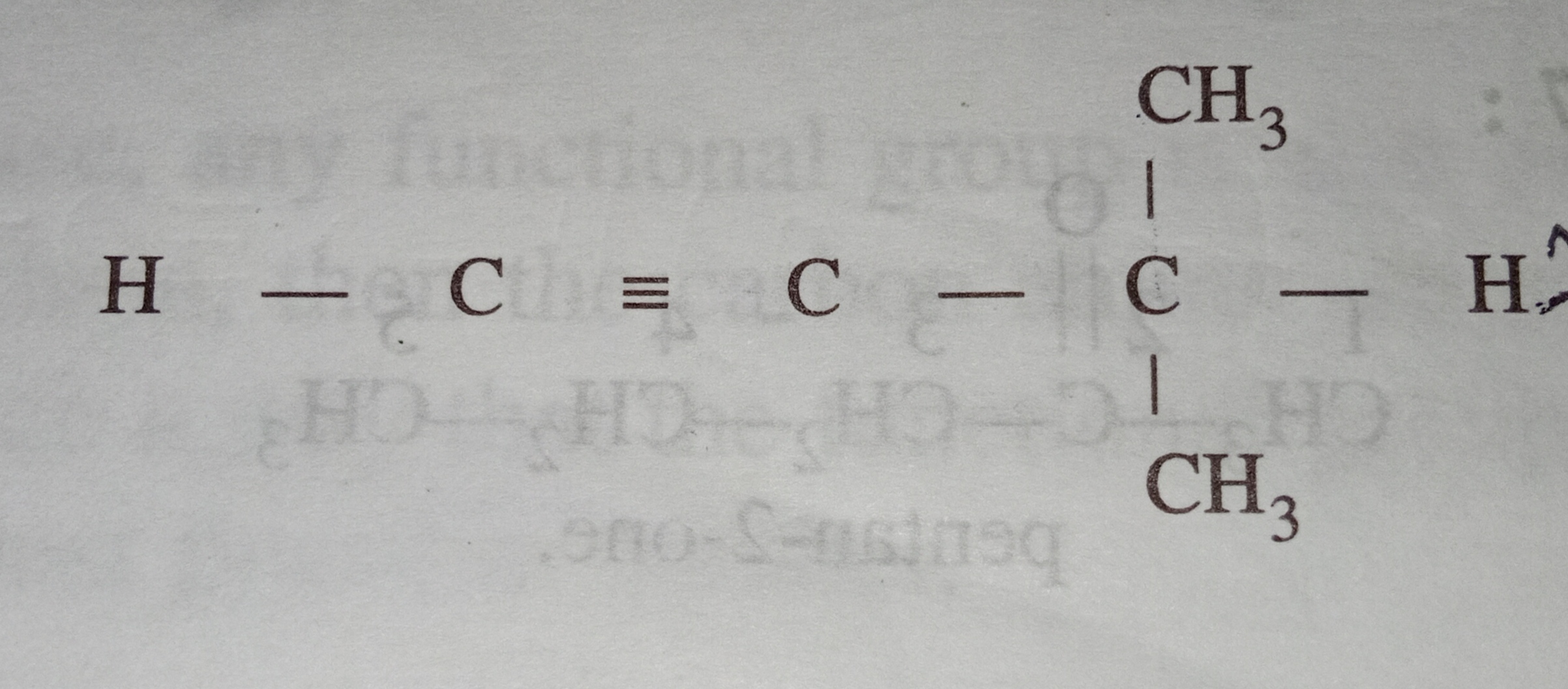
 hybridised ,how will it be classified?
hybridised ,how will it be classified?