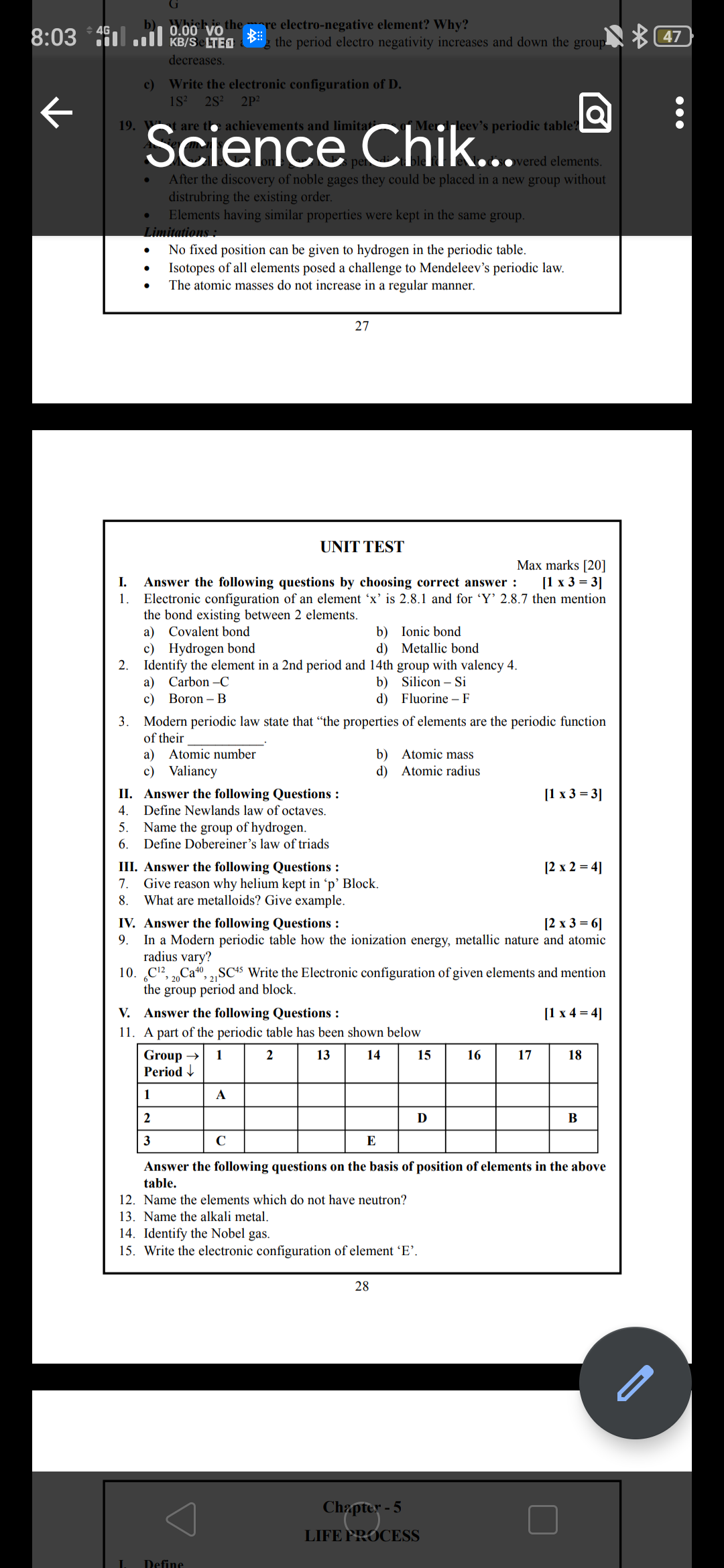
CBSE Class 10 Answered
Please write all the trends in groups and periods of periodic table with reasons (each reason should be atleast of 3 -4 lines)
Asked by sajal2402 | 09 Mar, 2019, 10:50: AM
Trends in periodic table:
Atomic size:
Atomic size increases down the group.
As the electron is added further away from the nucleus and electron shielding effect prevents the electrons being attracted toward the nucleus and thus the atomic size increases.
In a period atomic size decreases on moving left to right.
As the electrons are added in the same shell and nucleus attracts the electrons more strongly and this results in the decrease in atomic radius.
Electron affinity:
Electron affinity increases across a period from left to right in a periodic table.
As in a period from left right, the atomic size decreases and due to this the electron move closer to the nucleus, thus increasing the electron affinity.
Electron affinity decreases down the group as the atomic size increases. Due to which the electron is added further away from the nucleus and electron affinity decreases.
Electronegativity:
It is the tendency of an atom to attract bonding pair of electrons.
From left to right across a period of elements, electro negativity increases.
From top to bottom down a group, electro negativity decreases.
Answered by Varsha | 09 Mar, 2019, 04:20: PM
Application Videos
Concept Videos
CBSE 10 - Chemistry
Asked by dhumalchhaya13 | 26 May, 2022, 11:05: PM
CBSE 10 - Chemistry
Asked by kanchanbalpande82 | 12 Apr, 2022, 11:01: AM
CBSE 10 - Chemistry
Asked by kanchanbalpande82 | 12 Apr, 2022, 11:01: AM
CBSE 10 - Chemistry
Asked by waghmaresheetal78 | 27 Dec, 2021, 05:02: PM
CBSE 10 - Chemistry
Asked by ksheera36 | 03 Jun, 2021, 08:35: PM
CBSE 10 - Chemistry
Asked by vungtsaniyanthan | 16 May, 2021, 06:32: PM
CBSE 10 - Chemistry
Asked by sinhagopalakumara | 01 May, 2021, 08:16: PM
CBSE 10 - Chemistry
Asked by advssdrall | 26 Mar, 2021, 07:43: AM
CBSE 10 - Chemistry
Asked by shettyshrinidhi271 | 07 Jan, 2021, 05:04: PM
CBSE 10 - Chemistry
Asked by adipadmakarri | 05 Dec, 2020, 06:41: PM