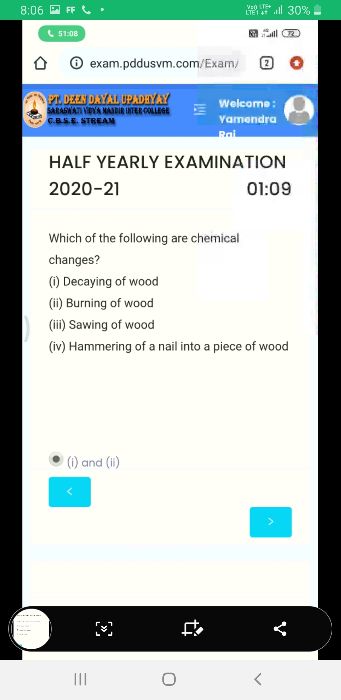
CBSE Class 9 Answered
Please tell me the clear definition of latent heat of vaporisation.
Asked by Harshit Saini | 13 Apr, 2015, 04:11: PM
The energy required to change the phase of a substance is known as a latent heat. Latent means hidden. When the phase change is from solid to liquid it is termed as the latent heat of fusion, and when the phase change is from liquid to a gas, it is known as the latent heat of vaporisation.
When we boil water to evaporate 100 °C, all the given heat is absorbed by the liquid. This absorbed heat is called latent heat of vaporization.
Answered by Arvind Diwale | 15 Apr, 2015, 09:42: AM
Application Videos
Concept Videos
CBSE 9 - Chemistry
Asked by rubinapathan228 | 29 Jun, 2023, 05:45: PM
CBSE 9 - Chemistry
Asked by yforyt3672 | 09 Apr, 2023, 07:37: PM
CBSE 9 - Chemistry
Asked by pranavtamboli65.9 | 02 Aug, 2022, 08:12: PM
CBSE 9 - Chemistry
Asked by ritapriya126 | 20 Apr, 2022, 04:01: PM
CBSE 9 - Chemistry
Asked by renukaramaswamyr | 08 Sep, 2021, 12:32: PM
CBSE 9 - Chemistry
Asked by gpranithasri | 27 Jul, 2021, 01:23: PM
CBSE 9 - Chemistry
Asked by suhanivasi007 | 04 May, 2021, 05:52: PM
CBSE 9 - Chemistry
Asked by pk36229905 | 06 Apr, 2021, 07:38: AM
CBSE 9 - Chemistry
Asked by anubhavev | 30 Oct, 2020, 06:35: PM