CBSE Class 12-science Answered
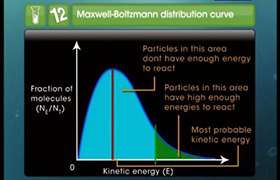
 Please send solution of 2 questions on chemical kinetics
Please send solution of 2 questions on chemical kinetics
Asked by sudhanshubhushanroy | 17 Nov, 2017, 09:41: AM
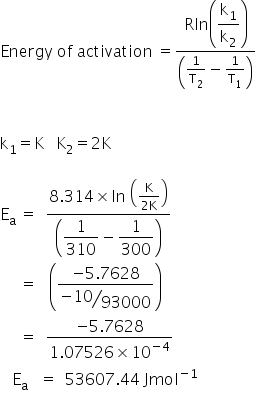
Answered by Varsha | 17 Nov, 2017, 11:01: AM
Concept Videos
CBSE 12-science - Chemistry
Asked by mishraridhi2020 | 23 Jun, 2022, 09:16: AM
CBSE 12-science - Chemistry
Asked by imtiyazmulla68 | 22 Mar, 2018, 08:25: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 22 Jun, 2016, 10:39: AM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 22 Jun, 2016, 09:56: AM