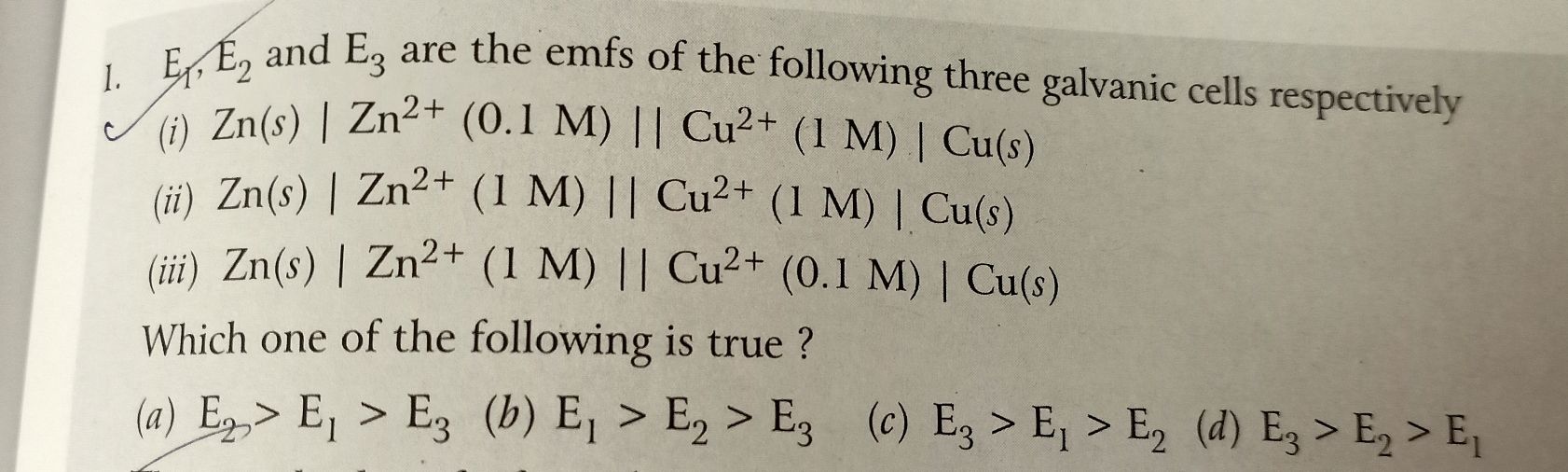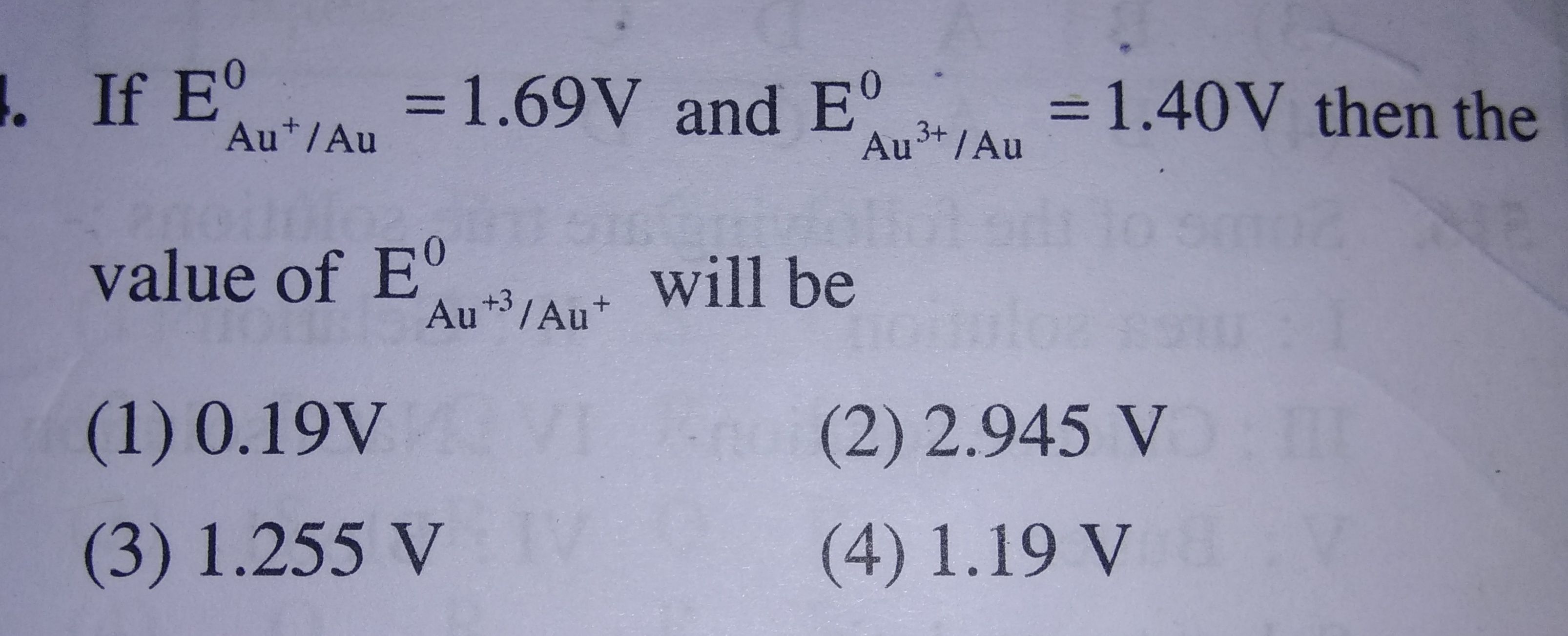CBSE Class 12-science Answered
Please provide the soln with full explanation and also which metal can be taken as anode or cathode and wh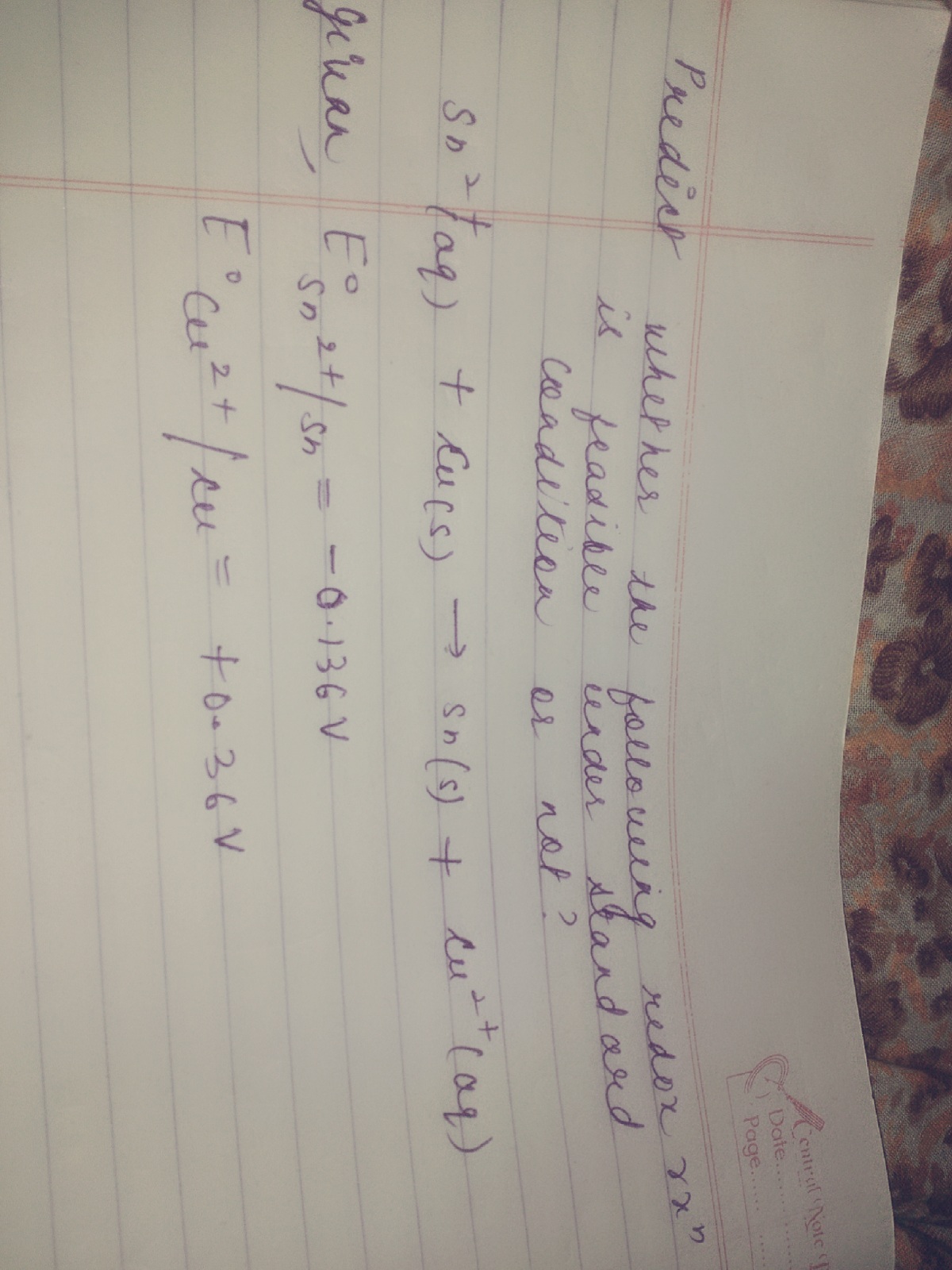
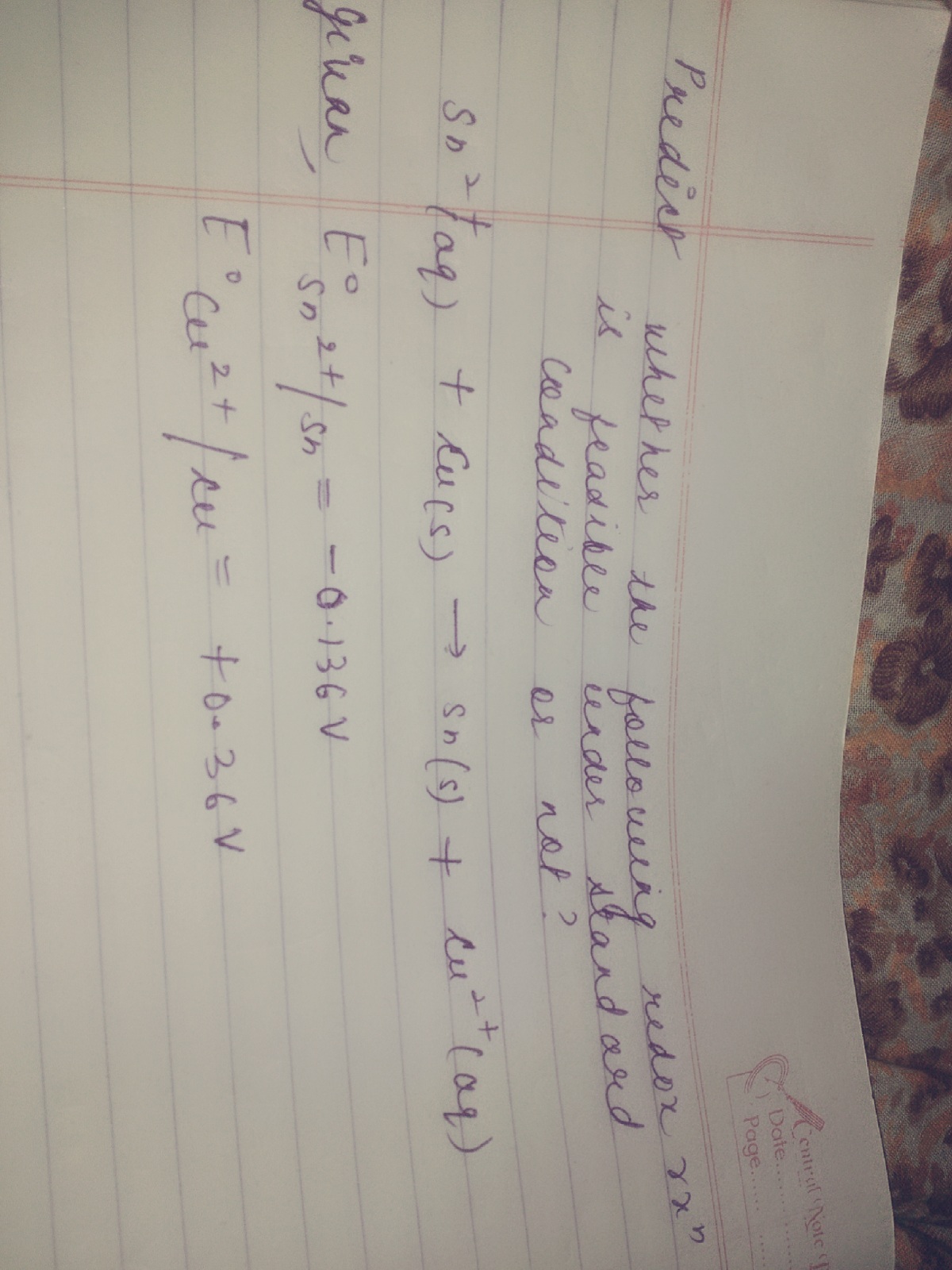
Asked by ayushi | 08 Jul, 2016, 10:01: AM
- In practice, the reaction will proceed if the E° value of the reaction is greater than + 0.40V.
- An equation with a more positive E° value will reverse one which is less positive.
Hence if an Sn(s) / Sn2+ (aq) cell and a Cu(s) / Cu2+ (aq) cell are connected:
- The appropriate equations are: Cu2+ (aq) + 2e¯ → Cu(s) ; E° = +0.36V Sn2+ (aq) + 2e¯ → Sn(s) ; E° = -0.136 V
- The half reaction with the more positive E° value is more likely to work
- It gets the electrons it needs by reversing the half reaction with the lower E° value
- So, Cu2+ (aq) ——> Cu(s) and Sn(s) ——> Sn2+ (aq)
- The overall reaction is Cu2+ (aq) + Sn(s) ——> Sn2+ (aq) + Cu(s)
- The cell voltage will be the difference in E° values ... (+0.34) - (-0.14) = + 0.48V
- Hence, Sn2+ (aq) + Cu (s) → Sn (s) + Cu2+(aq) is not feasible.
Answered by Prachi Sawant | 08 Jul, 2016, 04:17: PM
Concept Videos
CBSE 12-science - Chemistry
Asked by rajpatil | 19 Jun, 2020, 07:12: PM
CBSE 12-science - Chemistry
Asked by piyushkhariyal | 06 Mar, 2020, 10:36: PM
CBSE 12-science - Chemistry
Asked by prakriti12oct | 15 Feb, 2020, 08:21: PM
CBSE 12-science - Chemistry
Asked by prakriti12oct | 11 Nov, 2019, 12:09: AM
CBSE 12-science - Chemistry
Asked by govtsecschoolnayaganv051 | 05 Nov, 2019, 04:50: PM
CBSE 12-science - Chemistry
Asked by Balbir | 21 Aug, 2019, 09:14: PM
CBSE 12-science - Chemistry
Asked by ankitathapliyal097 | 24 Jul, 2019, 10:05: PM
CBSE 12-science - Chemistry
Asked by govtsecschoolnayaganv051 | 26 Jun, 2019, 02:18: PM
CBSE 12-science - Chemistry
Asked by jhajuhi19 | 01 May, 2019, 09:03: PM
CBSE 12-science - Chemistry
Asked by pardeepkumar2281 | 26 Sep, 2018, 09:00: PM