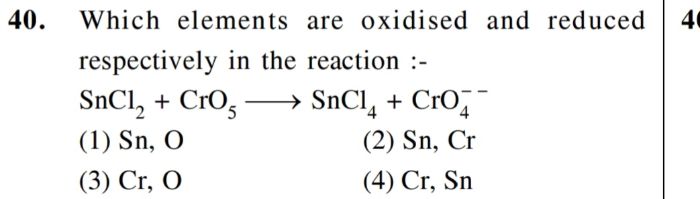CBSE Class 12-science Answered
Please provide a simple explanation regarding the electrode potential of the d block elements. What is t2g?
Asked by poojakamde3 | 01 Dec, 2017, 12:17: PM
M→M2+
M2+→M3+
The above equations signifies the standard electrode potentials of some half–cells involving 3d-series of transition elements and their ions in aqueous solution,
Electrode potential for a Mn+/M half-cell is a measure of the tendency for the reaction, Mn+(aq) + ne– → M(s)
Look out at this series:
Standard electrode potentials for 3d-elements

The above transition series expalin the trends of d- block elements in which the negative values of E° for the first series of transition elements (except for Cu2+/ Cu ) indicate that metals should liberate hydrogen from dilute acids i.e., the reactions.
Answered by Ramandeep | 01 Dec, 2017, 06:39: PM
Concept Videos
CBSE 12-science - Chemistry
Asked by anubhutiupadhaya | 27 Feb, 2024, 04:28: PM
CBSE 12-science - Chemistry
Asked by basib61203 | 08 Feb, 2024, 06:03: PM
CBSE 12-science - Chemistry
Asked by ABHILASHA | 04 Mar, 2021, 02:26: AM
CBSE 12-science - Chemistry
Asked by ghoshmahadev037 | 20 Sep, 2020, 11:45: AM
CBSE 12-science - Chemistry
Asked by mufeedatvp2000 | 20 Apr, 2020, 02:53: PM
CBSE 12-science - Chemistry
Asked by patra04011965 | 25 Sep, 2019, 10:22: PM
CBSE 12-science - Chemistry
Asked by patra04011965 | 22 Sep, 2019, 01:42: PM
CBSE 12-science - Chemistry
Asked by jain.pradeep | 30 Aug, 2019, 08:09: AM
CBSE 12-science - Chemistry
Asked by Debdulal | 29 Aug, 2019, 04:46: PM
CBSE 12-science - Chemistry
Asked by Debdulal | 29 Aug, 2019, 04:44: PM