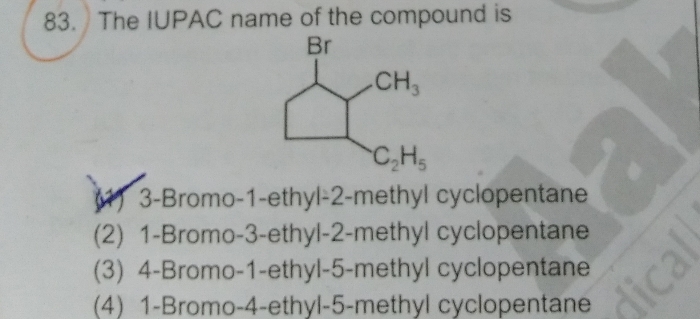
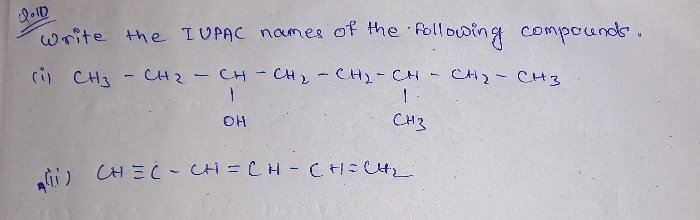
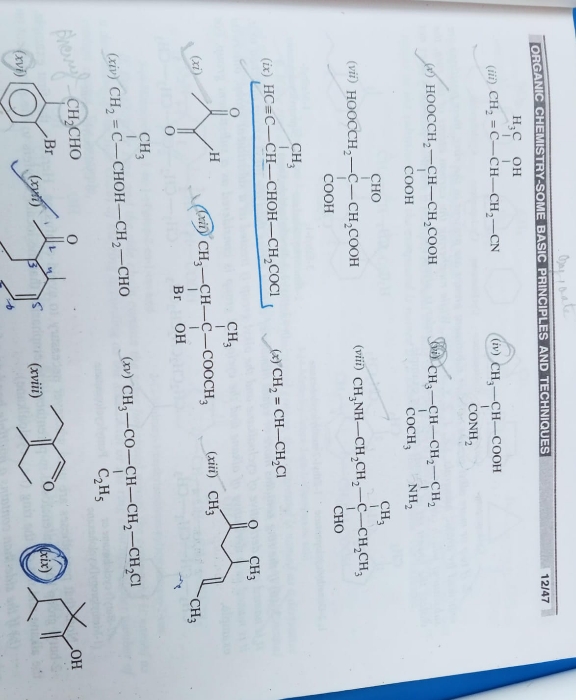
CBSE Class 11-science Answered
Resonance is a key component of valence bond theory and arises when no single conventional model using only single, double or triple bonds can account for all the observed properties of the molecule.
Resonance occurs because of the overlap of orbitals. Double bonds are made up of pi bonds, formed from the overlap of 2p orbitals. The electrons in these pi orbitals spread over more than two atoms, and hence are delocalized.
Both paired and unshared electrons may be delocalized, but all the electrons must be conjugated in a pi system.
All resonance structures for the same molecule must have the same sigma framework (sigma bonds form from the "head on" overlap of hybridized orbitals).
The hybrid structure is defined as the superposition of the resonance structures. A benzene ring is often shown with a circle inside a hexagon (in American texts) rather than alternating double bonds. The latter example misrepresents the electronic structure. Bonds with broken bond orders are often displayed as double bonds with one solid and one dashed line.
Resonance structures are imaginary. They represent extremes of electron location.
In general chemistry the concept of resonance was introduced through inorganic anions such as NO3-, NO2-, ClO4-, SO4-2, CO3-2.
Rule #1: Resonance structures must have the same number of electrons. (You should prove it to yourself by counting them).
Rule #2: All resonance structures must be valid Lewis dot structures.
Rule #3: The hybridization does not change between resonance structures.
Rule #4: The positions of atoms are the same in all structures.
Rule #5: Resonance structures do not have to be equivalent.
The curved arrow shows “movement” of a pair of electrons. It’s an extremely useful accounting system that lets us keep track of changes in bonding and also in charge. Since electron pairs are present either in bonds or in lone pairs, there are really only four combinations of “moves”.