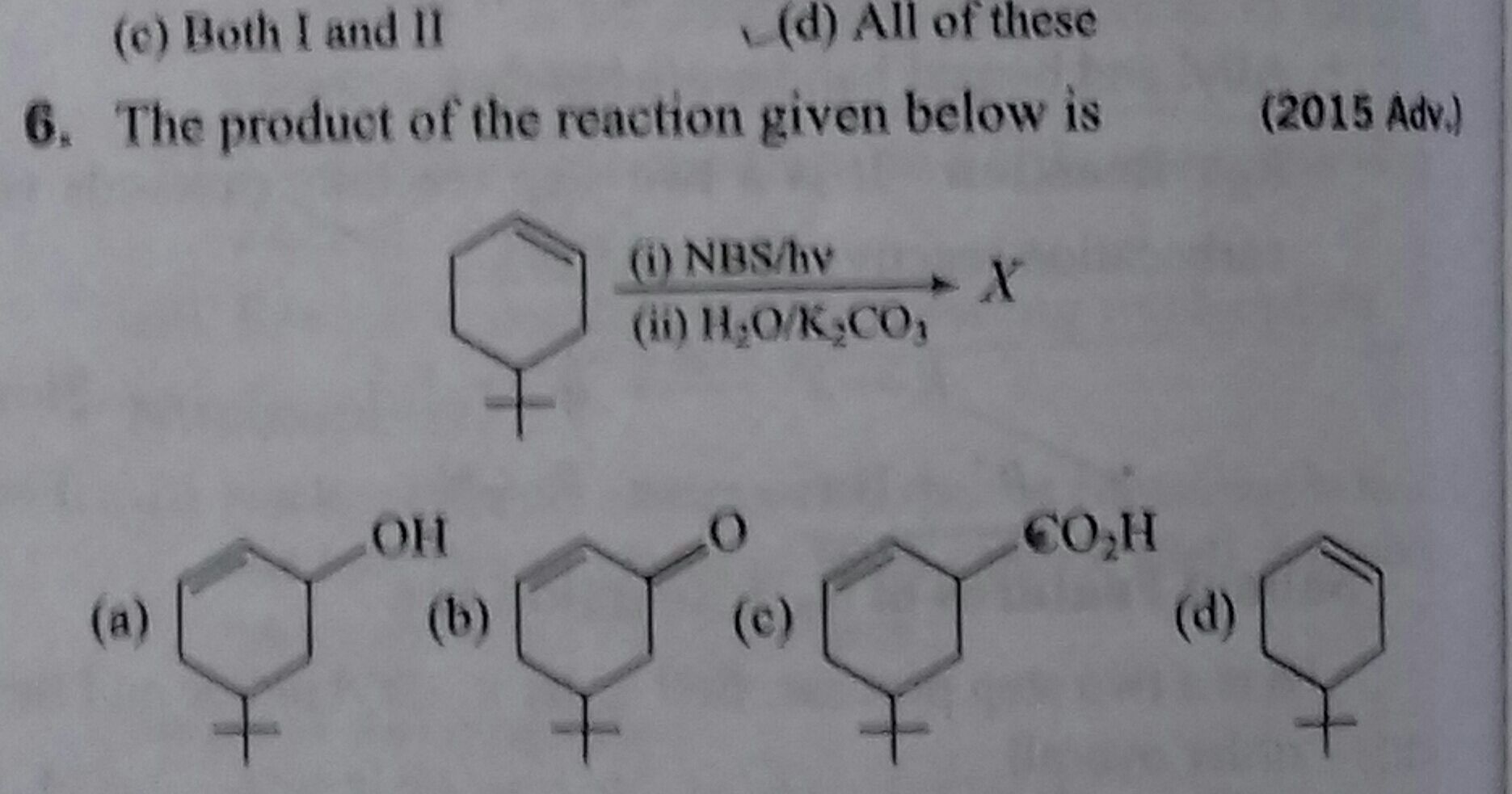JEE Class main Answered
Please help me solving electrophilic aromatic substitution reaction's ques.
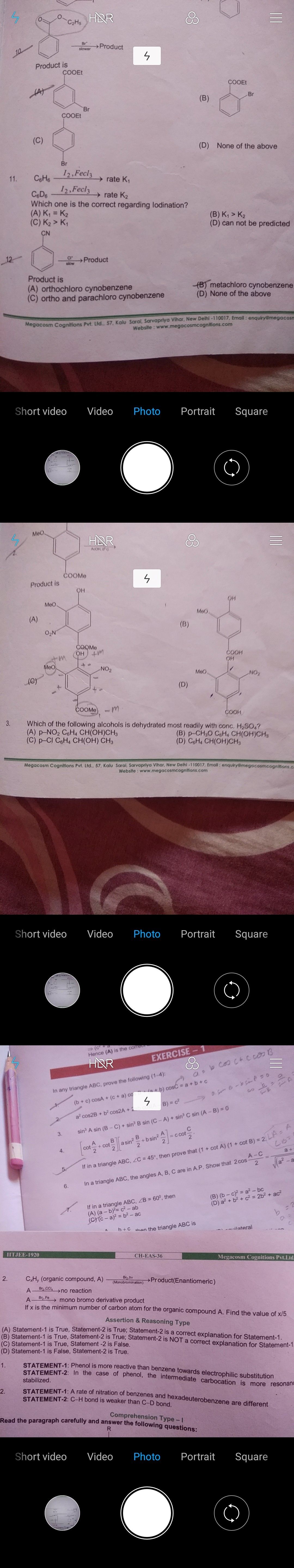
Ques. 11 of first pic.
Ques. 3 of 2nd pic.
Ques. 2 of third pic.
Please solve all three with detailed mechanism.
It won't take much time.
Please help.
ThanQ!
Asked by jhajuhi19 | 29 Mar, 2020, 11:19: AM
Ans. 10
For ethyl benzoate, the aromatic ring has a -CO2CH2CH3 group attached which is an electron withdrawing group. Therefore the electrophilic aromatic substitution reaction will occur at the meta position giving 3-nitrophenyl ethanoate.
Ans. 11
A rate of Iodination of benzenes and hexadeuteriobenzene are different because C-D bond is stronger than C-H bond since mass of deuterium is twice that of hydrogen which gives strength in bonding.
Hence, K1>K2
Ans.12
Similarly as Ans.10, CN is an deactivating group hence the product obtained is metachlorocynobenzene.
Answered by Ramandeep | 29 Mar, 2020, 09:03: PM
JEE main - Chemistry
Asked by muppanenicharitha | 14 Apr, 2024, 08:23: PM
JEE main - Chemistry
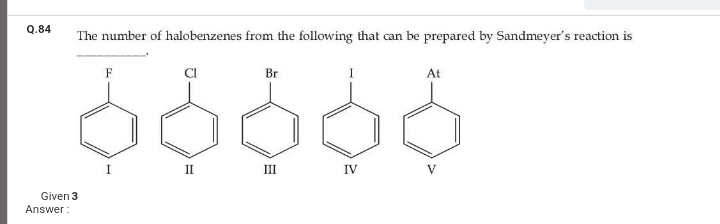
Asked by gourishettikrishna | 25 Jan, 2024, 10:01: PM
JEE main - Chemistry
Asked by hemeshsaini2005 | 11 Oct, 2021, 06:13: PM
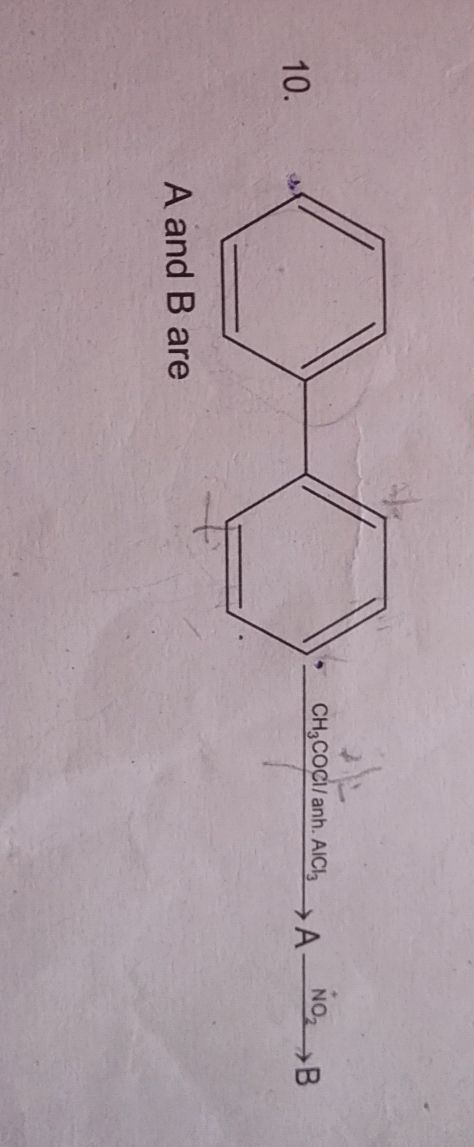
JEE main - Chemistry
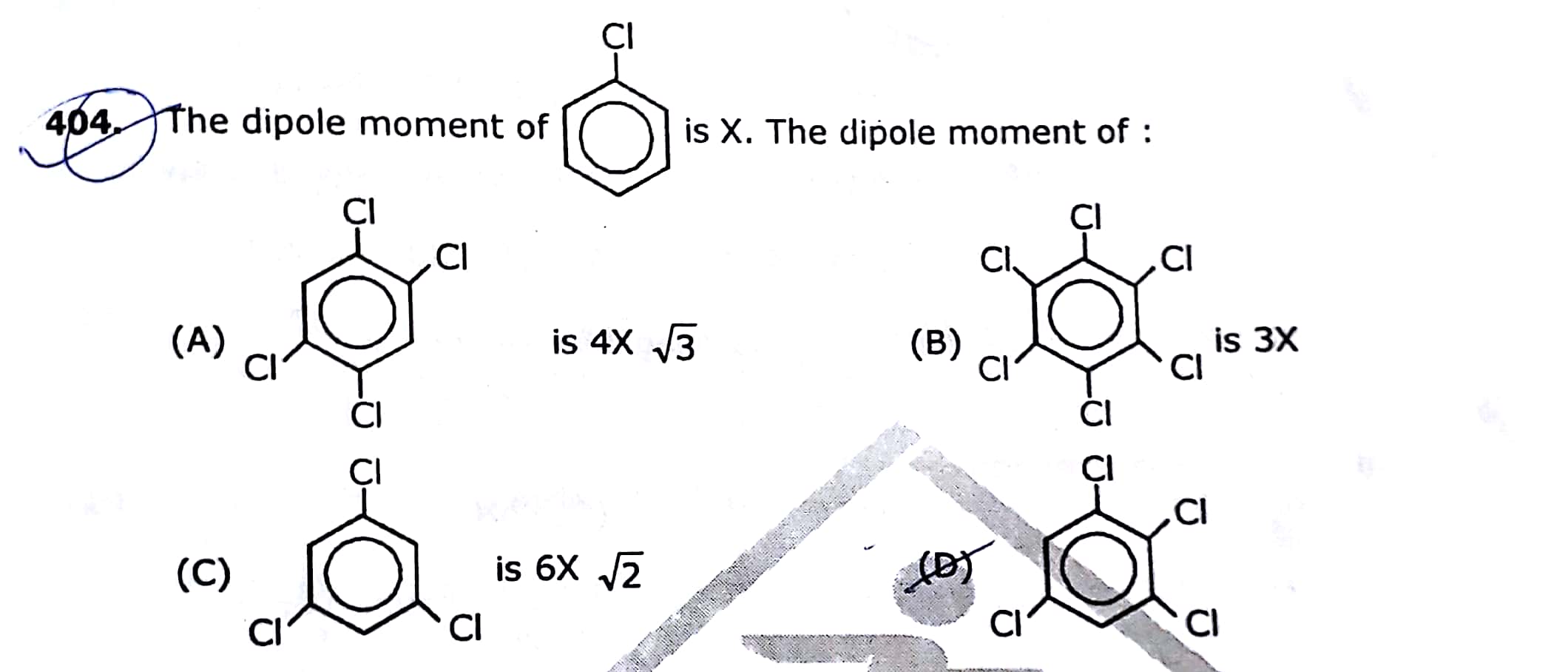
Asked by yasharthshankar | 30 Jun, 2020, 11:13: PM
JEE main - Chemistry
Asked by mdfaraz1182 | 09 Apr, 2020, 11:10: AM
JEE main - Chemistry
Asked by jhajuhi19 | 29 Mar, 2020, 11:19: AM
JEE main - Chemistry
Asked by vidyavikram10 | 29 Mar, 2020, 11:09: AM
JEE main - Chemistry
Asked by rsudipto | 24 Dec, 2018, 09:02: AM