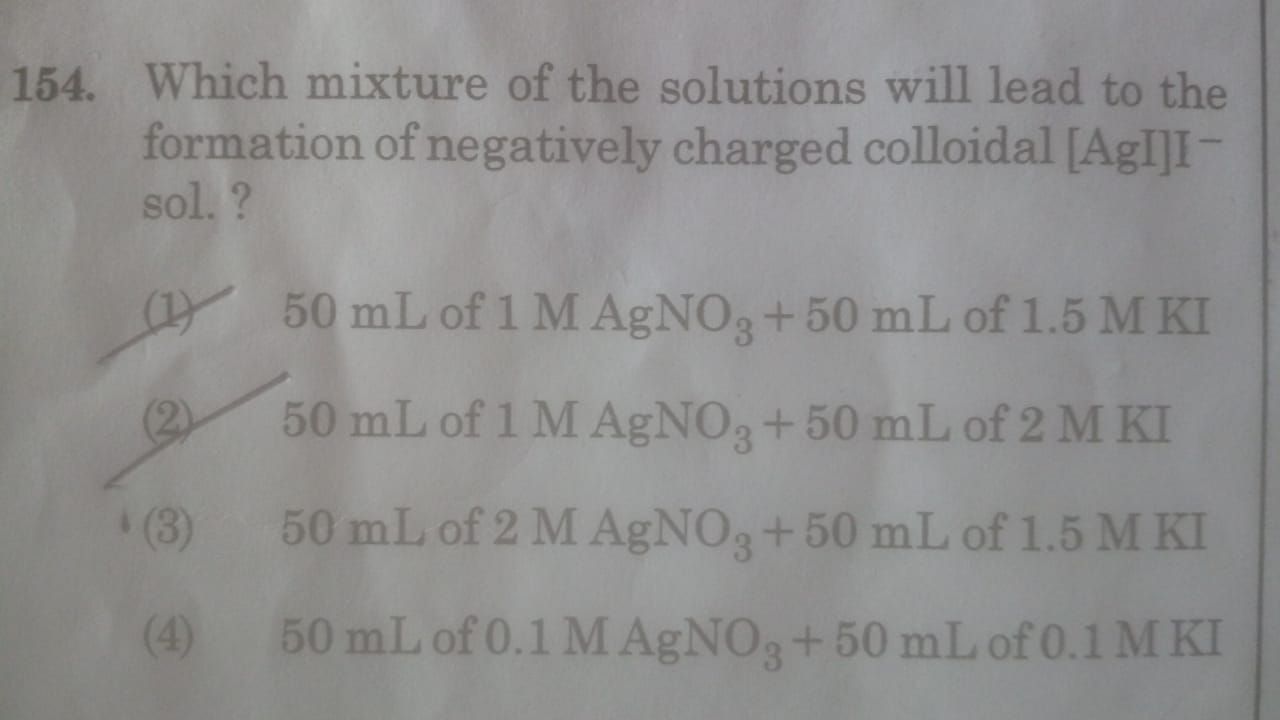CBSE Class 12-science Answered
please explain the concept of charge on colloidal particles due to preferential adsorption of ions
Asked by | 02 Feb, 2012, 09:18: PM
The colloidal particles have a tendency to preferentially adsorb a particular type of ions from the solution. A colloidal particle usually adsorbs those ions which are in excess and are common to its own lattice. This preferential adsorption of a particular type of ions imparts a particular type of charge to colloidal particles.
For example, when a ferric hydroxide sol is prepared by the hydrolysis of ferric chloride in warm water, the colloidal particles of Fe(OH)3 formed have a tendency to adsorb preferentially the Fe3+ ions present in the solution. This is because Fe3+ ions are common to the lattice of Fe(OH)3 particle. The Fe3+ ions thus adsorbed impart positive charge to the colloidal particles present in the sol.
Answered by | 03 Feb, 2012, 09:59: AM
Application Videos
Concept Videos
CBSE 12-science - Chemistry
Asked by mallavaramchandrika6 | 29 Sep, 2021, 05:27: PM
CBSE 12-science - Chemistry
Asked by Akshij Nanda | 11 Mar, 2021, 10:21: AM
CBSE 12-science - Chemistry
Asked by sivakapuganti1 | 26 Aug, 2020, 08:57: PM
CBSE 12-science - Chemistry
Asked by spoorthysaienelluri | 23 May, 2020, 11:07: AM
CBSE 12-science - Chemistry
Asked by shreemuvijihari | 18 May, 2020, 06:29: PM
CBSE 12-science - Chemistry
Asked by vermahitesh124 | 12 May, 2020, 07:58: AM
CBSE 12-science - Chemistry
Asked by yogeshsulakh | 07 Feb, 2020, 09:28: AM
CBSE 12-science - Chemistry
Asked by tlb2bpartner | 21 Aug, 2019, 10:28: PM
CBSE 12-science - Chemistry
Asked by ntg432000 | 22 May, 2019, 08:10: PM
CBSE 12-science - Chemistry
Asked by kripanjalihimansu | 01 Mar, 2019, 04:16: PM