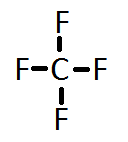CBSE Class 11-science Answered
The Lewis structure model generally follows the octet rule and provides a framework to understand covalent bonding. Lewis structures represent valence electrons as dots and bonding electrons as lines. Lewis structures do not represent inner electrons; only valence electrons are shown.
Here is a step-by-step procedure for writing valid Lewis structures for any given molecular formula:
- Count the total number of valence electrons by summing the group numbers of all the atoms. If there is a net positive charge, subtract that number from the total electron count. If there is a net negative charge, add that number to the total electron count.
- Draw single bonds to form the desired connectivity.
- Add lone pairs and multiple bonds, keeping the octet rule in mind.
- Add formal charges as needed.
Level 1 (basic)
1. Add up all the valance electrons of the atoms involved. ex CF4
So C has 4 and F has 7 (x4 we have 4Fs) = 32 valence electrons
2. You need to pick the central atom. This is usually easy, this atom will be surrounded by the others. Never H.
So C will be surrounded by F's.
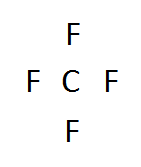
3. Now we create our skeleton structure by placing bonds in. A bond is a dash that represents 2 electrons.