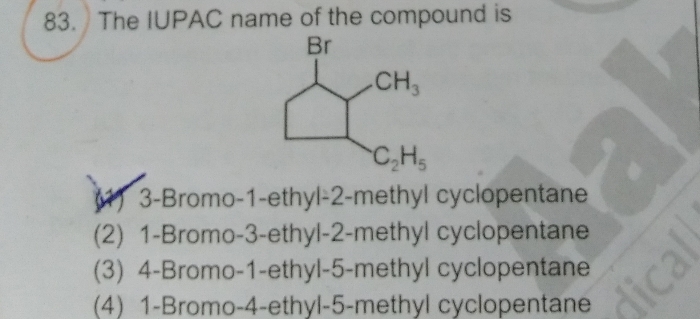
CBSE Class 11-science Answered
Please explain equivalent concept used to solve stoichiometry problem
Asked by | 02 Jun, 2008, 09:07: PM
Equivalent concept is similar to mole concept i.e in any chemical reaction the reactants react in same equivalents to give same equivalents of products.
BaCl2+Al2[SO4]3 --->BaSO4 + AlCl3
a b 0 0 [Before reaction]
0 b-a a a [after rection]
Where a and b are the number of equivalents. ( Number of equivalents = weight/(equivalent weight) )
Answered by | 17 Jun, 2008, 04:59: PM
Application Videos
Concept Videos
CBSE 11-science - Chemistry
Asked by rhythmdraco42 | 22 Apr, 2024, 10:43: PM
CBSE 11-science - Chemistry
Asked by hm6561889 | 15 Apr, 2024, 07:45: AM
CBSE 11-science - Chemistry
Asked by manikandanragul1 | 11 Apr, 2024, 09:02: AM
CBSE 11-science - Chemistry
Asked by jayag1298 | 08 Apr, 2024, 03:14: PM
CBSE 11-science - Chemistry
Asked by omniscientnjf2021 | 07 Apr, 2024, 10:18: PM
CBSE 11-science - Chemistry
Asked by ansh.skulkarni1158 | 07 Apr, 2024, 11:03: AM
CBSE 11-science - Chemistry
Asked by nikhithaguguloth14 | 29 Mar, 2024, 08:15: PM
CBSE 11-science - Chemistry
Asked by josephineanto1960 | 28 Mar, 2024, 12:50: PM
CBSE 11-science - Chemistry
Asked by sumedhasingh238 | 27 Mar, 2024, 11:04: PM
CBSE 11-science - Chemistry
Asked by neet2025targetgo | 25 Mar, 2024, 10:13: AM