NEET Class neet Answered
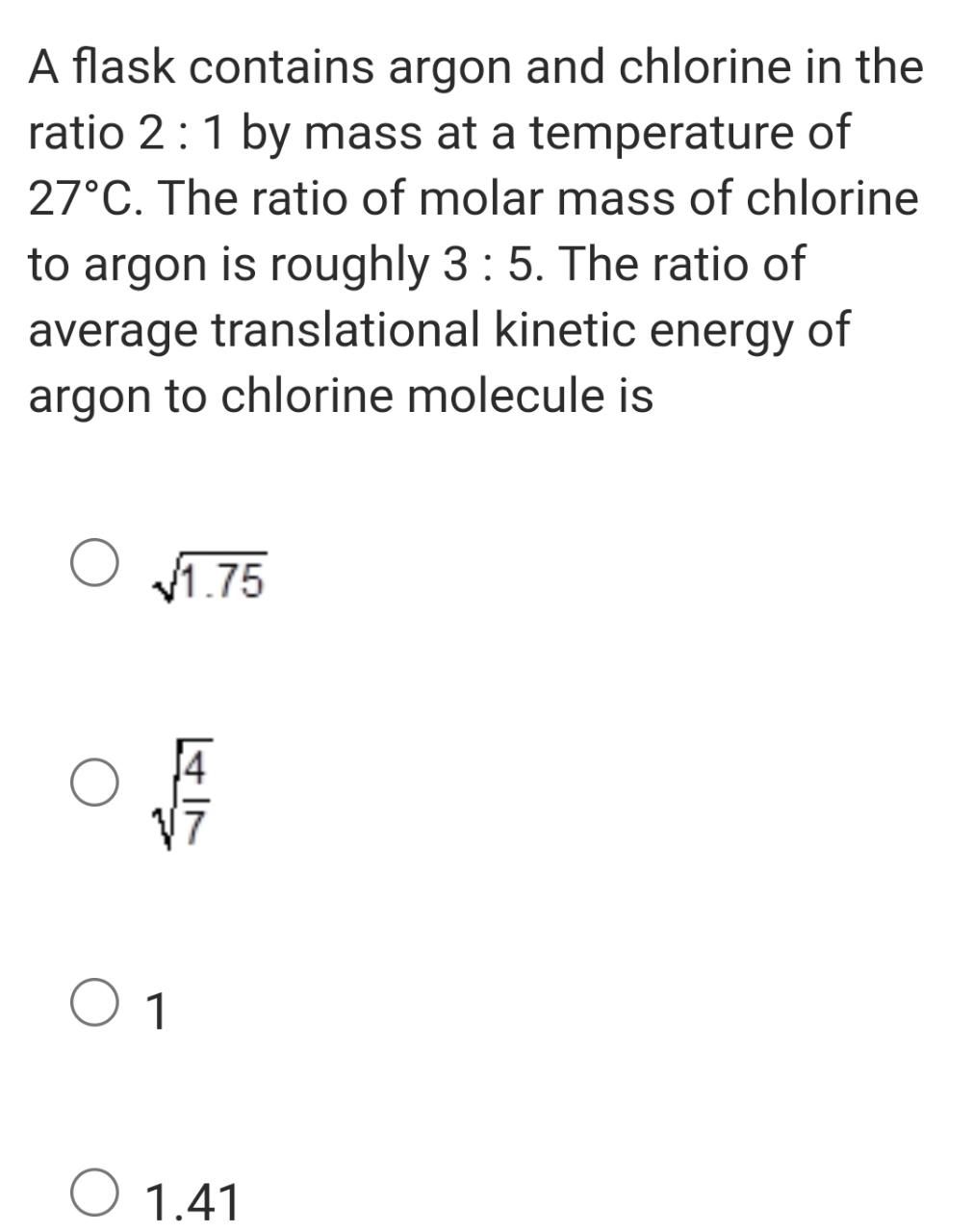
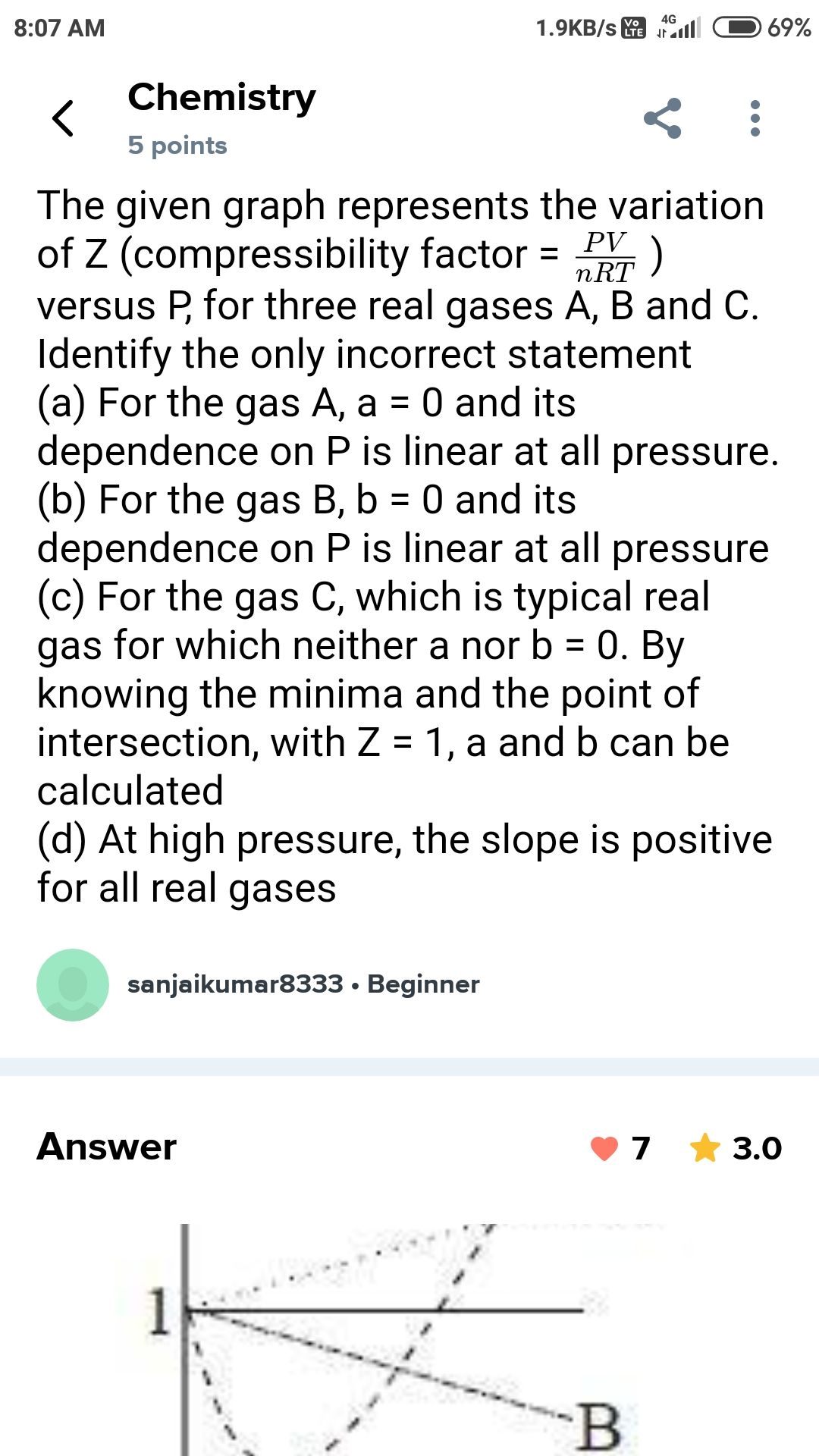
please answer this

Asked by Prashant DIGHE | 03 Mar, 2020, 10:06: PM
Given:
For He,
V1= 200 ml
P1 = 0.66 atm
V2 = 400 ml
P2 = ?
By using Boyle's law,
P1V1 = P2V2
0.66×200 = P2 × 400
P2 = 0.33 atm
For O2
V1= 400 ml
P1 = 0.52 atm
V2 = 400 ml
P2 = ?
By using Boyle's law,
P1V1 = P2V2
0.52×400 = P2 × 400
P2 = 0.52 atm
Partial pressure of He and O2 is 0.33 and 0.52 respectively.
Answered by Varsha | 04 Mar, 2020, 11:00: AM
NEET neet - Chemistry
Asked by raomayankup83 | 15 Apr, 2024, 07:46: PM
NEET neet - Chemistry
Asked by gopikakannan27 | 13 Jan, 2022, 05:39: AM
NEET neet - Chemistry
Asked by jhajuhi19 | 19 Aug, 2021, 08:38: PM
NEET neet - Chemistry
Asked by kowsalyamouli | 06 Oct, 2020, 04:08: PM
NEET neet - Chemistry
Asked by nssharma001969 | 03 Jun, 2020, 01:54: AM
NEET neet - Chemistry
Asked by prakriti12oct | 25 May, 2020, 12:15: AM
NEET neet - Chemistry
Asked by jhajuhi19 | 10 May, 2020, 08:24: AM
NEET neet - Chemistry
Asked by anushka.kulkarni02022002 | 19 Mar, 2020, 09:31: AM
NEET neet - Chemistry
Asked by Prashant DIGHE | 03 Mar, 2020, 10:09: PM
NEET neet - Chemistry
Asked by Prashant DIGHE | 03 Mar, 2020, 10:06: PM