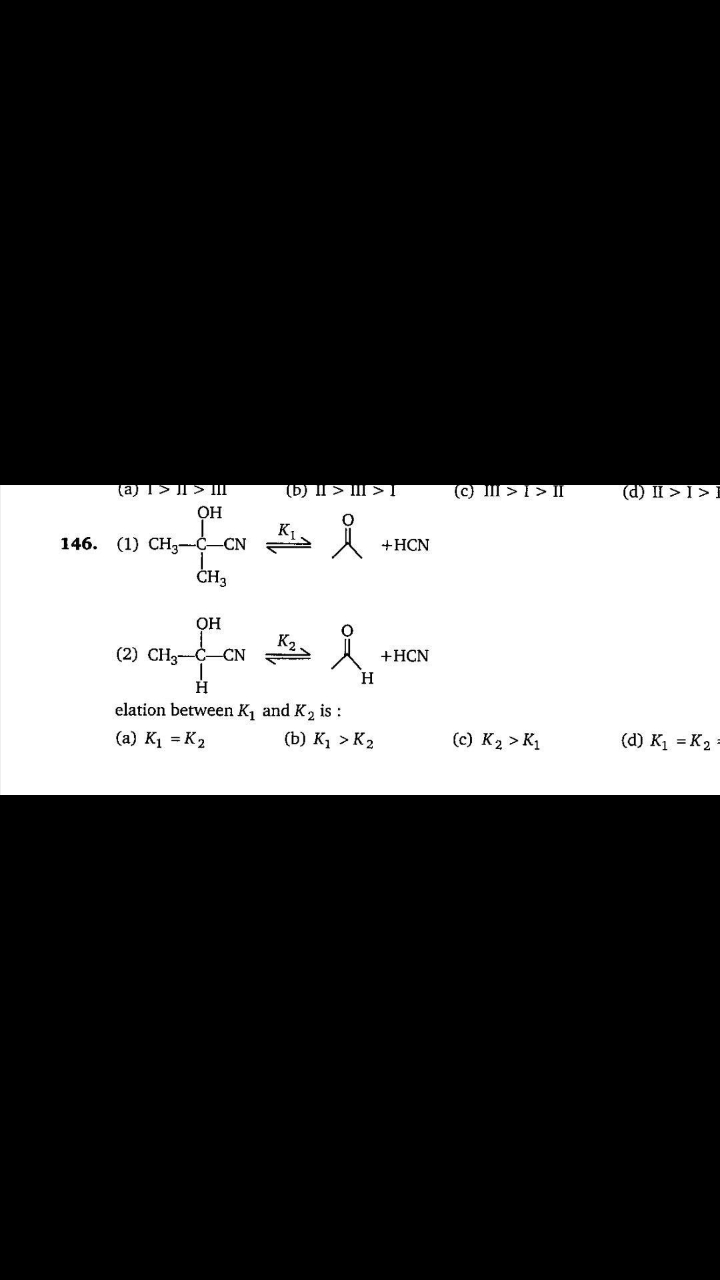
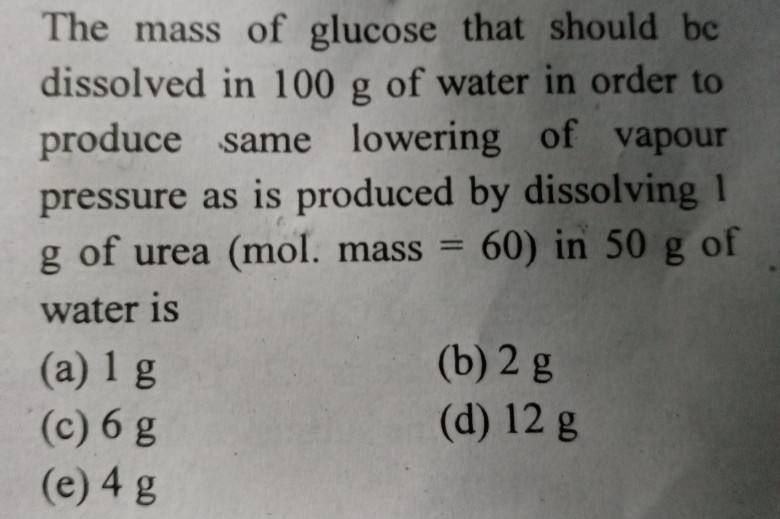NEET Class neet Answered
please answer this
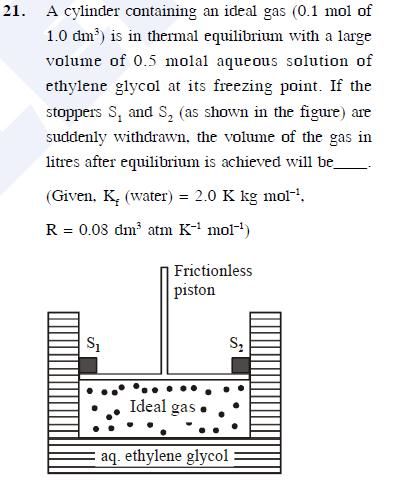
Asked by Prashant DIGHE | 28 Feb, 2020, 10:01: PM
Given:
Freezing point depression constant Kf = 2 K
m = 0.5 m
ΔTf = Kf × m
= 2× 0.5
ΔTf = 1
Tinitial = 272 K
n = 0.1 mol
V = 1 dm3
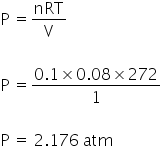
After realising piston,
P1V1 = P2V2
2.176 × 1 = 1 × V2
V2 = 2.176 dm3
Answered by Varsha | 01 Mar, 2020, 06:57: PM
Concept Videos
NEET neet - Chemistry
Asked by 8239682116rahul | 10 Apr, 2024, 01:48: PM
NEET neet - Chemistry
Asked by ramadevisupriya5678 | 28 Mar, 2024, 02:18: PM
NEET neet - Chemistry
Asked by myindiaisbad | 17 Jun, 2022, 11:17: AM
NEET neet - Chemistry
Asked by bhaveshkaria31 | 30 May, 2022, 09:26: PM
NEET neet - Chemistry
Asked by rautganesh2255 | 01 Jul, 2021, 09:32: AM
NEET neet - Chemistry
Asked by NituBarman192 | 01 Jun, 2021, 10:22: PM
NEET neet - Chemistry
Asked by bhagirathdangi12345 | 12 Feb, 2021, 01:42: PM
NEET neet - Chemistry
Asked by akashmanu09 | 08 Jan, 2021, 10:21: AM
NEET neet - Chemistry
Asked by arnavvidudala20050 | 17 May, 2020, 03:07: PM