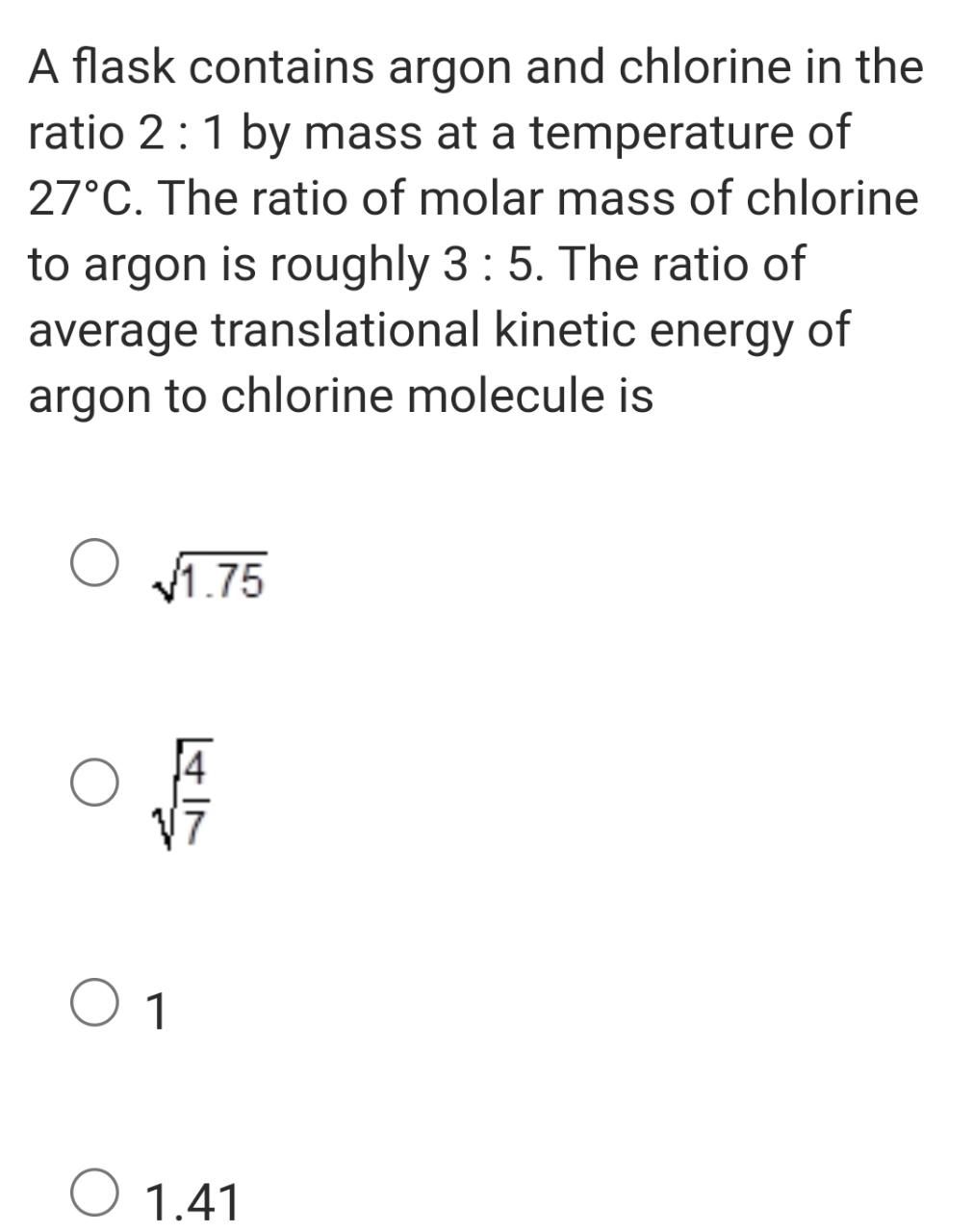
NEET Class neet Answered
please answer this

Asked by Prashant DIGHE | 03 Mar, 2020, 10:00: PM
Option (2) is correct.
Given:
For bulb A,
Volume, VA = 100 ml
Pressure, =PA
For Bulb B,
Volume, =VB
Pressure, PB = 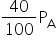
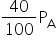
Using Boyle's law,
PAVA = PBVB
PA × 100 = 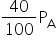 × VB
× VB
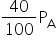 × VB
× VB VB = 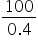
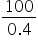
VB = 250 ml
Before opening stopcock, volume of gas in bulb B must be (250-100) = 150 ml
Answered by Varsha | 04 Mar, 2020, 09:47: AM
NEET neet - Chemistry
Asked by raomayankup83 | 15 Apr, 2024, 07:46: PM
NEET neet - Chemistry
Asked by gopikakannan27 | 13 Jan, 2022, 05:39: AM
NEET neet - Chemistry
Asked by jhajuhi19 | 19 Aug, 2021, 08:38: PM
NEET neet - Chemistry
Asked by kowsalyamouli | 06 Oct, 2020, 04:08: PM
NEET neet - Chemistry
Asked by nssharma001969 | 03 Jun, 2020, 01:54: AM
NEET neet - Chemistry
Asked by prakriti12oct | 25 May, 2020, 12:15: AM
NEET neet - Chemistry
Asked by jhajuhi19 | 10 May, 2020, 08:24: AM
NEET neet - Chemistry
Asked by anushka.kulkarni02022002 | 19 Mar, 2020, 09:31: AM
NEET neet - Chemistry
Asked by Prashant DIGHE | 03 Mar, 2020, 10:09: PM
NEET neet - Chemistry
Asked by Prashant DIGHE | 03 Mar, 2020, 10:06: PM