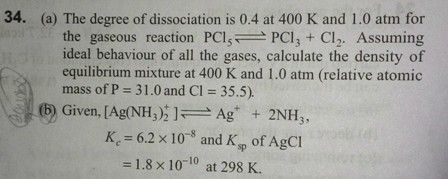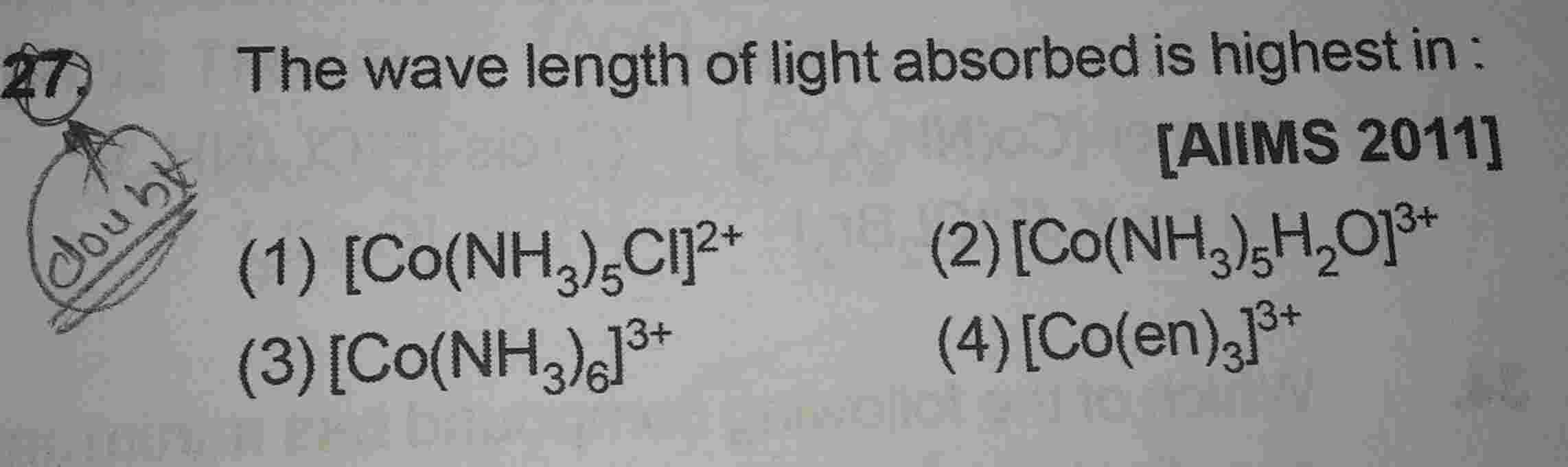NEET Class neet Answered
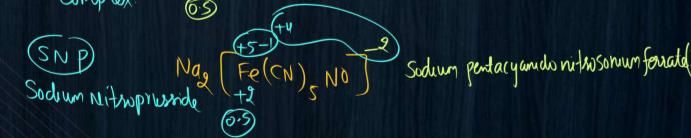
please answer this

Asked by Prashant DIGHE | 29 Mar, 2020, 09:39: PM
As we know greater is the number of unpaired electrons, larger is the paramagnetism.
A. [Co(ox)2(OH)2]-
O.S. of Co :
x +(-2)×2+(-1)×2=-1
x - 6 = -1
x = +5
Co+5 = [Ar] 3d4
It has 4 unpaired electrons
Similarly,
B. Ti(NH3)6]3+ =Ti3+ = [Ar] 3d1 only one unpaired electron.
C. [V(gly)2(OH)2(NH3)2]+ = V+5 =[Ar] 3d0 no unpaired electrons.
D [Fe(en)(bby)(NH3)2]2+ = Fe2+= [Ar]3d6
But as bby and NH3 are strong field electrons hence pairing occurs resulting no unpaired electrons.
Hence from above information it is clar that complex [Co(ox)2(OH)2]- has highest number of unpaird electrons hence it show highest paramagnetism.
Answered by Ramandeep | 30 Mar, 2020, 11:35: AM
NEET neet - Chemistry
Asked by anandibastavade555 | 25 Mar, 2024, 10:01: AM
NEET neet - Chemistry
Asked by ykrish407 | 20 Sep, 2022, 07:25: PM
NEET neet - Chemistry
Asked by priscillaabram | 05 Apr, 2022, 10:02: AM
NEET neet - Chemistry
Asked by singhalakshat6 | 02 Mar, 2022, 07:28: PM
NEET neet - Chemistry
Asked by Prashant DIGHE | 06 Jun, 2020, 09:45: PM
NEET neet - Chemistry
Asked by Prashant DIGHE | 29 Mar, 2020, 09:39: PM
NEET neet - Chemistry
Asked by Prashant DIGHE | 28 Mar, 2020, 10:30: PM
NEET neet - Chemistry
Asked by patra04011965 | 21 Jul, 2019, 11:40: PM