ICSE Class 10 Answered
Please Answer
2500cc of oxygen was burnt with 600cc of ethane [C2H6]. Calculate the volumeof unused oxygen and the volume of carbon dioxide formed.
Asked by vprincesolomon07 | 06 Aug, 2018, 11:09: PM
2C2H6 +7O2 →4CO2 +6H2O
2V 7V 4V
Now from the equation, 2V of ethane reacts with = 7 V of oxygen
So, 600cc of ethane reacts with= 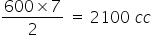
Hence, unused O2 is = 2500 - 2100 = 400 cc
From 2V of ethane = 4 V of CO2 is produced
So, 600cc of ethane will produce =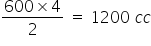 CO2
CO2
Answered by Ramandeep | 07 Aug, 2018, 11:32: AM
Concept Videos
ICSE 10 - Chemistry
Asked by ruchisharmatbn | 05 Mar, 2024, 06:40: PM
ICSE 10 - Chemistry
Asked by kundus458 | 07 Feb, 2024, 08:55: AM
ICSE 10 - Chemistry
Asked by matloobser | 07 Sep, 2023, 09:36: AM
ICSE 10 - Chemistry
Asked by dafk04.dp | 06 May, 2021, 06:22: PM
ICSE 10 - Chemistry
Asked by amit.clw4 | 15 Mar, 2021, 07:27: AM
ICSE 10 - Chemistry
Asked by amit.clw4 | 14 Mar, 2021, 08:12: AM
ICSE 10 - Chemistry
Asked by ravi.solaabhi | 17 Oct, 2020, 10:11: AM
ICSE 10 - Chemistry
Asked by payalagrawal1724 | 28 Jun, 2020, 07:22: PM
ICSE 10 - Chemistry
Asked by vijay.prag | 29 Dec, 2019, 08:07: PM
ICSE 10 - Chemistry
Asked by Dsangayy | 08 May, 2019, 07:11: PM






