CBSE Class 11-science Answered
pl experts ans as soon as possible
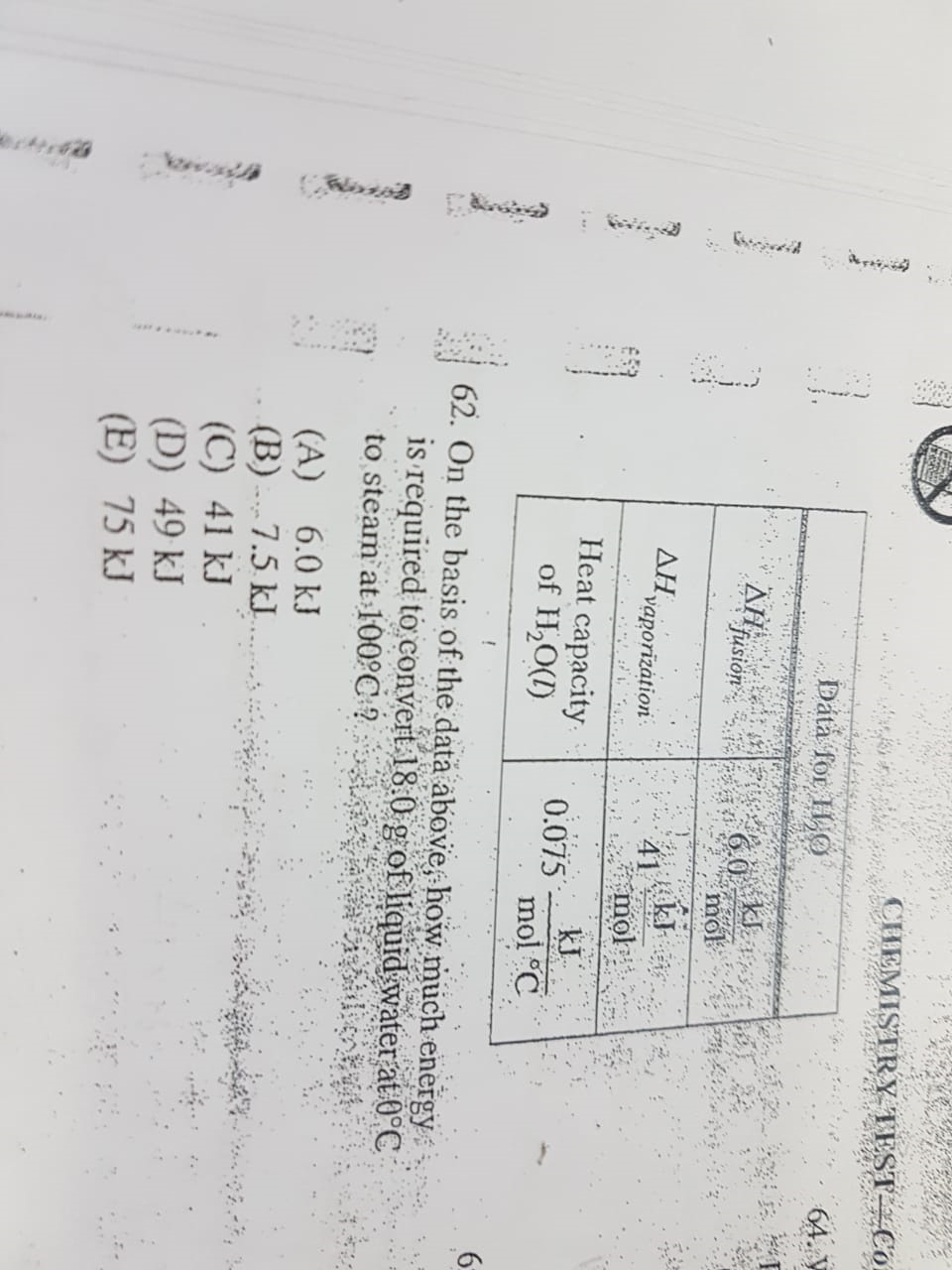
Asked by jain.pradeep | 14 Apr, 2019, 12:33: AM
Heat Capacity of water(C) = 0.075 KJ/mol oC
= 0.075 KJ/18goC
=0.00416 KJ/goC
Mass(m) = 18g
ΔT =100 oC
Q=mcΔT
=18×0.00416×100
=7.5 KJ
Answered by Ravi | 15 Apr, 2019, 03:55: PM
Concept Videos
CBSE 11-science - Chemistry
Asked by kjay0981 | 13 Dec, 2020, 03:45: PM
CBSE 11-science - Chemistry
Asked by jain.pradeep | 14 Apr, 2019, 12:33: AM
CBSE 11-science - Chemistry
Asked by Atulcaald | 25 May, 2018, 12:31: AM
CBSE 11-science - Chemistry
Asked by gganga | 13 Apr, 2018, 06:34: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 13 Aug, 2014, 01:38: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 15 Jun, 2016, 05:22: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 13 Aug, 2014, 02:12: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 15 Jun, 2016, 05:22: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 13 Aug, 2014, 02:32: PM





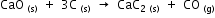 The heats of formation of CaO, CaC2 and CO are -151.6, -14.2 and -26.4 kcal respectively.
The heats of formation of CaO, CaC2 and CO are -151.6, -14.2 and -26.4 kcal respectively.