CBSE Class 10 Answered
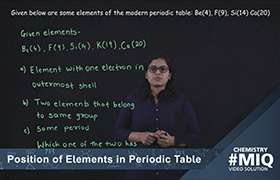
Periodic Classification of elements
Asked by SSHHII | 05 Jun, 2009, 10:58: PM
Electronic configuration of atoms depends on two things:
1)Capacity of shell which is given by 2 n2 . For eg. for first shell it is 2(1)2 =2, for 2nd it is 8, for 3rd 18 and so on.
2)It depends on the order in which it gets filled in subshells(s,p,d,f) of the shells. Theses subshells have varied capabilities and electron's get filled in them in accordance with aufbau's and hund's multiplicity rule. Thus we can obtain configuration of any element.
So various configurations are obtained following above rules.
Answered by | 07 Jun, 2009, 10:37: AM
Application Videos
Concept Videos
CBSE 10 - Chemistry
Asked by dhumalchhaya13 | 26 May, 2022, 11:05: PM
CBSE 10 - Chemistry
Asked by kanchanbalpande82 | 12 Apr, 2022, 11:01: AM
CBSE 10 - Chemistry
Asked by kanchanbalpande82 | 12 Apr, 2022, 11:01: AM
CBSE 10 - Chemistry
Asked by waghmaresheetal78 | 27 Dec, 2021, 05:02: PM
CBSE 10 - Chemistry
Asked by ksheera36 | 03 Jun, 2021, 08:35: PM
CBSE 10 - Chemistry
Asked by vungtsaniyanthan | 16 May, 2021, 06:32: PM
CBSE 10 - Chemistry
Asked by sinhagopalakumara | 01 May, 2021, 08:16: PM
CBSE 10 - Chemistry
Asked by advssdrall | 26 Mar, 2021, 07:43: AM
CBSE 10 - Chemistry
Asked by kavithasenthil148 | 06 Feb, 2021, 07:09: PM
CBSE 10 - Chemistry
Asked by mina6poonam | 21 Jan, 2021, 04:57: PM