CBSE Class 12-science Answered
NO2 group shows its effect only at the ortho para position why ?
Asked by Gishin Rajan | 15 Jan, 2016, 09:54: AM
In Nitrobenzene, the resonance structures have positive charges around the ring system:
 Every functional group shows its maximum effect on the ortho and para positions only as these two positons are in the plane of the ring unlike the meta position.
Every functional group shows its maximum effect on the ortho and para positions only as these two positons are in the plane of the ring unlike the meta position.

As we know that NO2 group is Electron withdrawing groups (EWG). It pulls the ring electrons towards itself. (See the Diagram).
Due to this pulling of electron; it decreases the electron density at ortho and para position of the ring (+ sign at o and p position). But, meta position remains unaffected during this movement of electron.
Answered by Hanisha Vyas | 15 Jan, 2016, 01:00: PM
Concept Videos
CBSE 12-science - Chemistry
Asked by roshanisharma200611 | 07 Feb, 2024, 01:18: PM
CBSE 12-science - Chemistry
Asked by surekhas66675 | 02 Sep, 2021, 05:17: PM
CBSE 12-science - Chemistry
Asked by nazimb0313 | 02 Sep, 2020, 09:34: AM
CBSE 12-science - Chemistry
Asked by mastertask199 | 13 May, 2020, 04:08: PM
CBSE 12-science - Chemistry
Asked by jain.pradeep | 03 Feb, 2020, 10:42: PM
CBSE 12-science - Chemistry
Asked by ajaysankhala051 | 11 Sep, 2019, 01:19: PM
CBSE 12-science - Chemistry
Asked by pragyachandraul03 | 23 Aug, 2019, 02:12: PM
CBSE 12-science - Chemistry
Asked by priadkonkar | 21 Jan, 2019, 08:52: PM
CBSE 12-science - Chemistry
Asked by rakeshraghav33 | 21 Jan, 2019, 02:49: PM
CBSE 12-science - Chemistry
Asked by Atulcaald | 16 May, 2018, 02:34: PM




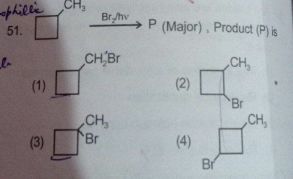
 Total No. of Mono Brominated product :-
Total No. of Mono Brominated product :-