CBSE Class 11-science Answered
My exam is tomorrow, please answer Fast . Thanks in advance ..
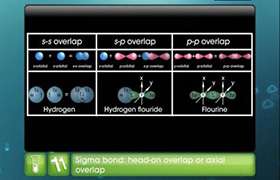
In NCERT TB pg 117 ,right side, 1st para , it has been given that in sp hybridisation of BeCl2 , "each of the sp hybridized orbital overlaps with the 2p orbital of chlorine axially and forms two Be-Cl sigma bonds . "
but , the elect. Config of chlorine is 1s2 2s2 2p6 3s2 3p5 . As per this , Be has to overlap axially with 3p orbital of chlorine . It cannot overlap with 2p as it it already filled . But the textbook has mentions that it overlaps with 2p .... Plz explain this .... Thanks in advance .
Asked by Saravanan | 19 Jun, 2014, 04:57: PM
- Ground state electronic configuration of Be: 1s22s2
- In the excited state one of the 2s-electrons is promoted to vacant 2p orbital.
- One 2s and one 2p orbital gets hybridised to fom two sp hybridised orbitals.
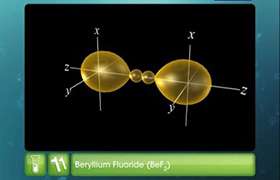
- Each Be-Cl bond arises by axial overlap of a sp hybrid orbital on beryllium with a 3p orbital on chlorine.
Answered by Vaibhav Chavan | 20 Jun, 2014, 11:55: AM
Concept Videos
CBSE 11-science - Chemistry
Asked by Trisha Gupta | 30 Oct, 2022, 05:36: PM
CBSE 11-science - Chemistry
Asked by ABHILASHA | 22 Aug, 2020, 04:39: AM
CBSE 11-science - Chemistry
Asked by kpbhake | 12 Mar, 2018, 11:45: AM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 08 Oct, 2014, 01:09: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 13 Jun, 2016, 02:26: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 11-science - Chemistry
What is the hybrid state of B in BF3, Al in AlCl3, Be in BeCl2, C in CO2 and C2H4; S in SO2 and SO3.
Asked by Topperlearning User | 08 Oct, 2014, 01:33: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 09 Oct, 2014, 09:30: AM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM


 on the basis of hybridisation
on the basis of hybridisation