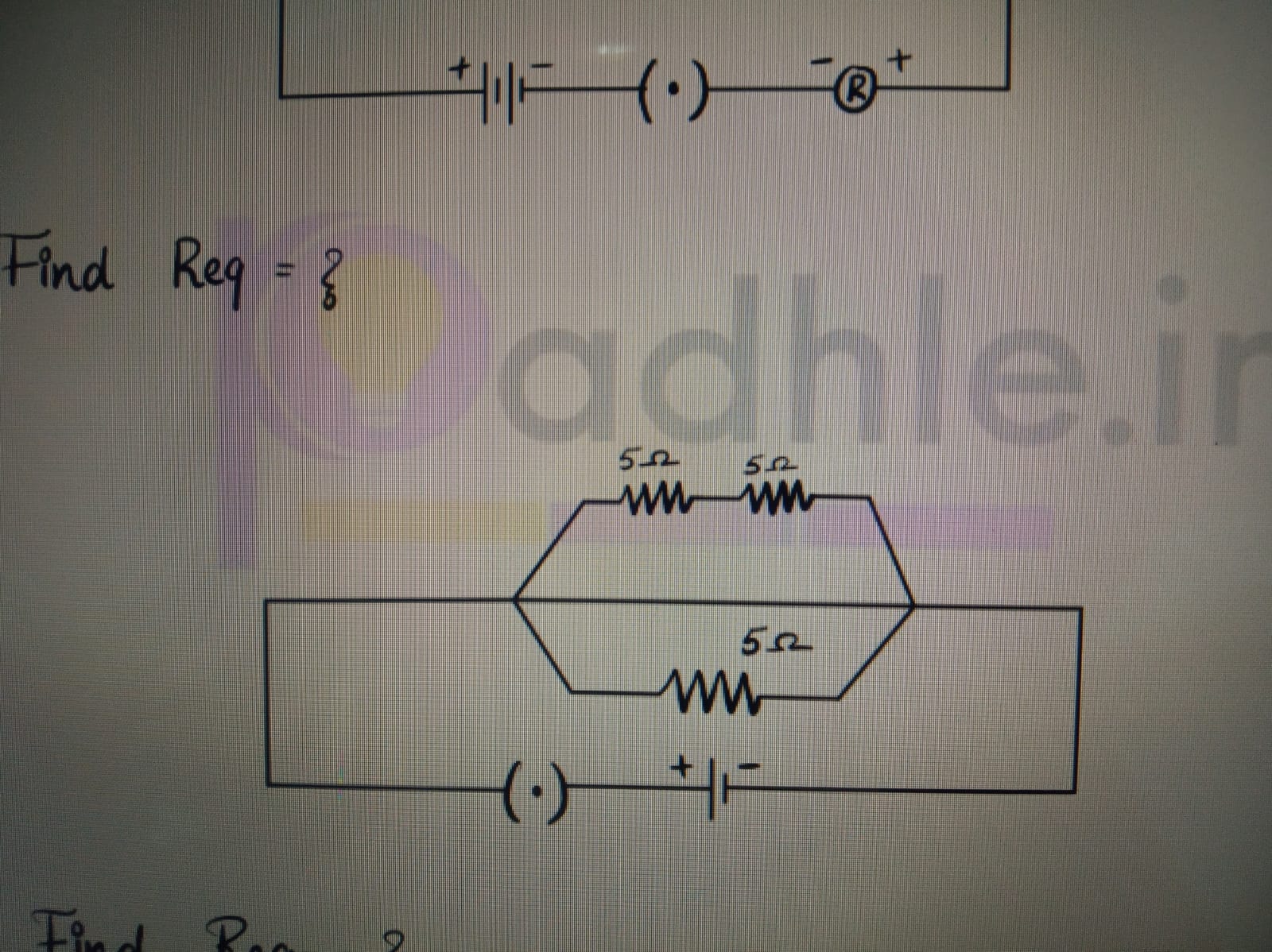
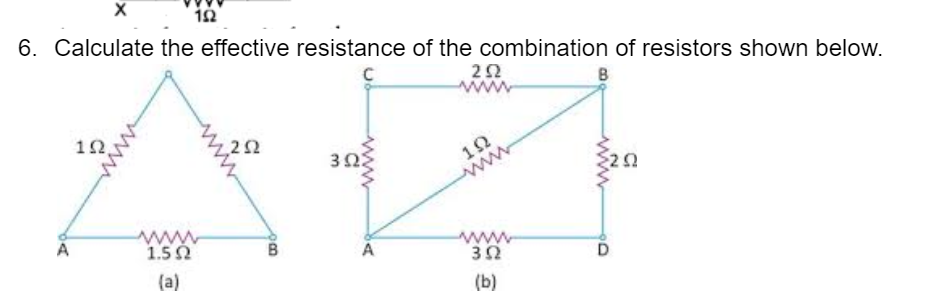
CBSE Class 10 Answered
My Calorimeter(Joules' apparatus) is equipped with a thermometer and it gives reading in celcius. So please tell me how to convert it into calorie or joule.
Asked by Manoj | 20 Jun, 2013, 07:38: PM
4186 joule of work is spent to produce the same amount of heat which could raise the temperature of one kg of water through 10C.
So, depending on how much water you have,
heat produced = mC(delta T)
where C is the specific heat of water called = 4.186J/g (degreeC)
delta T defines the change in temperature and m is the mass of water.
Also, 1 calorie = 4.184J, so once you will have the heat produced in joules, you can convert it to calories.
Answered by | 23 Jun, 2013, 06:02: AM
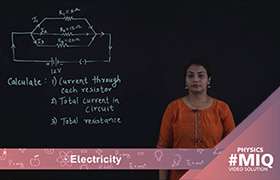
Application Videos
Concept Videos
CBSE 10 - Physics
Asked by khajannirwan | 27 Feb, 2024, 10:20: PM
CBSE 10 - Physics
Asked by saanviyadla | 24 Jan, 2024, 07:06: PM
CBSE 10 - Physics
Asked by kamalaranjanmohantymohanty5 | 06 Jan, 2024, 10:05: AM
CBSE 10 - Physics
Asked by nandhikasugumar | 05 Oct, 2023, 04:01: PM
CBSE 10 - Physics
Asked by daniya062008 | 02 Oct, 2023, 08:25: PM
CBSE 10 - Physics
Asked by prassanna.j | 03 Sep, 2023, 12:28: PM
CBSE 10 - Physics
Asked by prassanna.j | 03 Sep, 2023, 12:21: PM
CBSE 10 - Physics
Asked by prassanna.j | 03 Sep, 2023, 12:13: PM
CBSE 10 - Physics
Asked by prassanna.j | 03 Sep, 2023, 12:11: PM