CBSE Class 12-science Answered
Mass of sucrose C12H22O11 produced by mixing 84 gm of carbon, 12 gm of hydrogen and 56 L. O2 at 1 atm & 273 K according to given reaction, is
C(s) + H2(g) + O2(g) → C12H22O11(s)
Asked by Atulcaald | 19 May, 2018, 12:05: AM
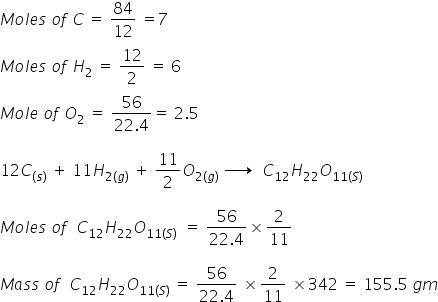
Answered by Ramandeep | 21 May, 2018, 10:17: AM
Concept Videos
CBSE 12-science - Chemistry
Asked by praveenk5480 | 31 May, 2021, 11:22: AM
CBSE 12-science - Chemistry
Asked by naikmamata688 | 02 Aug, 2020, 09:41: AM
CBSE 12-science - Chemistry
Asked by shahwajahat1604 | 03 Jun, 2020, 09:10: PM
CBSE 12-science - Chemistry
Asked by lovemaan5500 | 27 Nov, 2019, 12:23: PM
CBSE 12-science - Chemistry
Asked by Atulcaald | 19 May, 2018, 12:05: AM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 22 Jun, 2016, 07:58: AM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 22 Jun, 2016, 07:59: AM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 22 Jun, 2016, 08:00: AM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 22 Jun, 2016, 08:00: AM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM



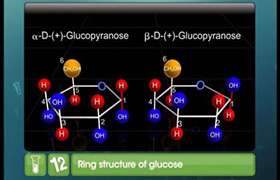
 (i) What are such isomers called? (ii) Can they be called enantiomers? Justify your answer. (iii) Draw the cyclic structure of isomer (A).
(i) What are such isomers called? (ii) Can they be called enantiomers? Justify your answer. (iii) Draw the cyclic structure of isomer (A).